Definition of hydrogel and hydrocolloid
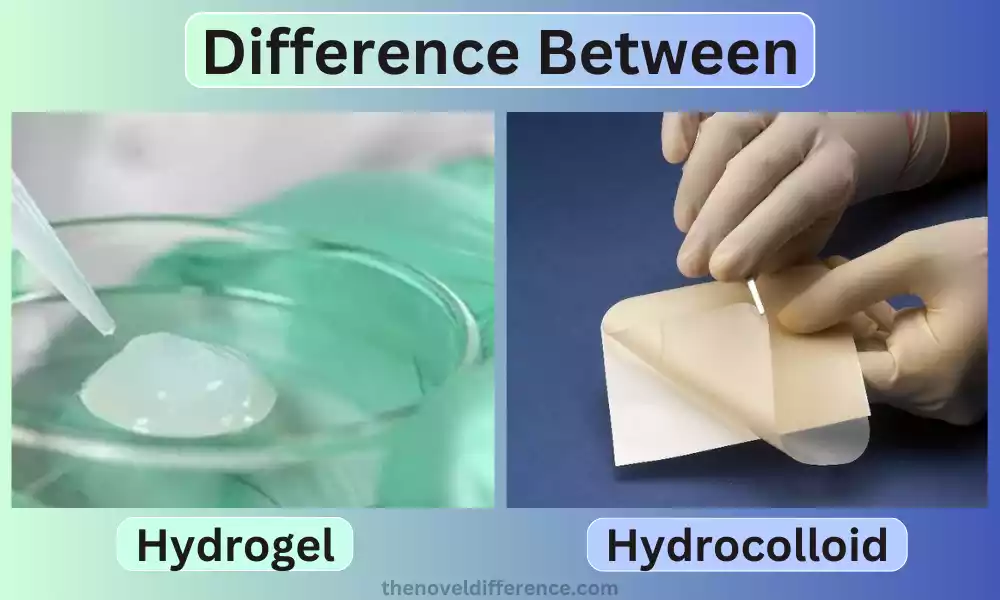
Hydrogel: Hydrogel refers to a three-dimensional network structure composed largely of water that forms into gel-like material, capable of retaining large quantities of fluid while remaining solid-like in consistency.
Hydrogels can either be natural or synthetic and possess different physical and chemical properties depending on their composition, typically known for high water retention and biocompatibility, mimicking certain aspects of biological tissues, mimicking drug delivery systems, tissue engineering, or contact lens applications.
Hydrogels also find applications within biomedical engineering, drug delivery systems tissue engineering as well as contact lens applications among many others.
Hydrocolloids: A hydrocolloid is a class of substances that can gel or thicken when immersed in water. These substances are usually polysaccharides such as pectin or agar, or proteins, like collagen or gelatin.
Hydrocolloids have many applications across industries, from food and beverage production to pharmaceutical research and personal care products.
Hydrocolloid dressings have long been recognized for their effective wound care capabilities and effectiveness at creating moist wound environments, managing exudate, protecting from contamination, and speeding healing. Furthermore, hydrocolloids’ adhesive qualities create an additional protective and supportive atmosphere around a wound’s site.
Importance and applications of hydrogel and hydrocolloid materials
Hydrogel:
Importance:
- Biocompatibility: Hydrogels are biocompatible materials, meaning that they tend to be well tolerated by living tissues. This makes them suitable for various biomedical applications, including tissue engineering, drug delivery systems, and implants.
- Water absorption and retention: Hydrogels can absorb and retain large amounts of water or biological fluids. This property is beneficial for applications such as wound dressings, contact lenses, and drug delivery systems.
- Structural similarity to biological tissues: The hydrated structure of hydrogels closely resembles that of biological tissues, making them suitable for applications requiring biomimetic properties.
Applications:
- Biomedical engineering: Hydrogels are used in tissue engineering to create scaffolds that support the growth and regeneration of cells and tissues.
- Drug delivery systems: Hydrogels can be used as carriers for controlled and targeted drug delivery, providing sustained release of therapeutic agents.
- Contact lenses: Soft contact lenses are often made from hydrogel materials due to their high water content and biocompatibility.
- Biosensing and diagnostic devices: Hydrogels have proven useful as biosensors and diagnostic devices by being customized to recognize certain biomarkers, making them effective tools in biosensing and diagnostic applications.
Hydrocolloid:
Importance:
- Moisture management: Hydrocolloids can absorb moisture from wounds or body surfaces while maintaining a moist environment. This promotes wound healing and prevents tissue desiccation.
- Exudate management: Hydrocolloid dressings are effective in managing exudate by absorbing it into the dressing, thereby reducing the risk of maceration and providing a conducive environment for wound healing.
- Protection against bacterial contamination: Hydrocolloid dressings create a barrier that protects wounds from external contaminants and bacteria, reducing the risk of infection.
- Adhesive properties: Hydrocolloid dressings have self-adhesive properties that allow them to adhere to the skin, providing secure and comfortable wound coverage.
Applications:
- Wound care and dressings: Hydrocolloid dressings are widely used for the management of chronic and acute wounds, including pressure ulcers, diabetic ulcers, and surgical wounds.
- Ostomy care products: Hydrocolloid-based ostomy appliances provide a secure and leak-proof seal around stoma sites, improving comfort and preventing skin irritation.
- Dental applications: Hydrocolloid materials are used in dentistry for impression-taking and as soft tissue management materials during dental procedures.
- Food Industry Applications: Hydrocolloids like pectin, agar, and carrageenan are used in food products as thickeners, stabilizers, and gelling agents to thicken, stabilize, and gel products.
Both hydrogel and hydrocolloid materials play crucial roles in various industries and have unique properties that make them valuable for specific applications, ranging from biomedical and pharmaceutical fields to wound care and food industry applications.
What Is Hydrogel?
Hydrogel is a three-dimensional network structure of water molecules interlaced into its network structure to form a semisolid or jellylike substance known as “hydrogel,” where 90-99% water forms its total composition.
Hydrogels are widely known for having such high water contents – from 90%-99% in most instances – and they make for ideal applications to store sensitive data without risk of leakage and theft.
Hydrogels stand out as unique materials due to their extraordinary capacity for absorbing and holding onto large volumes of liquid without losing their solid-like consistency, through a process called swelling.
Through swelling, water enters its network structure without dissolving or losing integrity – the hallmarks of successful hydrogel performance!

Hydrogels can be divided into two broad categories, natural hydrogels, and synthetic hydrogels:
Natural Hydrogels: Natural hydrogels are made up of materials obtained from biological sources such as polysaccharides such as agarose, alginate, or chitosan or proteins like gelatin or collagen. Such hydrogels offer biocompatibility and biodegradability similar to that found in biological tissue structures. Natural hydrogels have many biomedical applications, from tissue engineering and drug delivery systems to wound healing.
Synthetic Hydrogels: These artificial gels can be created through chemical reactions that polymerize monomers into crosslinked networks for crosslinking structures that mimic natural hydrogels in biomedical applications.
Synthetic hydrogels offer properties such as mechanical strength, swelling capacity, and degradation rate that can be modified for specific applications. Utilized extensively in biomedical research applications as well as drug delivery systems, contact lenses, and more, synthetic hydrogels have found widespread application throughout various sectors such as medicine delivery systems.
Hydrogels possess various notable characteristics and functions:
Hydrogels possess great swelling capacity: Their capacity to absorb and retain large volumes of water allows them to become swelling gels that can expand exponentially with every drop taken in, swelling in response. Their composition and crosslink density determines their degree of swelling capacity.
Mechanical Strength and Elasticity: Hydrogels exhibit various mechanical properties depending on their composition and crosslinking density, from soft to elastic or more rigid and stiff depending on composition or crosslink density.
Biocompatibility and degradability: Hydrogels can often be designed for safe biomedical applications thanks to their biocompatibility. With gradual degradation over time, hydrogels provide safe use.
Drug Delivery and Release: Hydrogels serve as effective carriers to safely deliver therapeutic agents into the body through controlled release or slow release, whether within or on its surface. Drug molecules can either be enclosed by or loaded onto its matrix for maximum effect.
Hydrogels mimic biological environments: Their water-rich nature closely resembles that of biological tissues, making hydrogels ideal for applications including cell culture, tissue engineering, and regenerative medicine.
Hydrogels are versatile materials with a high water content that find use across various fields – biomedical engineering, drug delivery, and tissue regeneration being just three such examples where their unique properties come into play.
What Is Hydrocolloid?
Hydrocolloid is a group of substances with the capability of thickening when exposed to liquid, such as water. The name derives from hydro, meaning water, and colloids which refer to suspended materials in other media such as polysaccharides like pectin or proteins (gelatin or collagen) being suspended as micelles within them.
Hydrocolloids usually come from natural sources like polysaccharides like pectin or proteins such as gelatin or collagen being dispersed within other media and forming thickened gels within them when exposed to liquid.
Hydrocolloids possess special characteristics that make them suitable for numerous uses in diverse settings. When exposed to moisture or water, hydrocolloids undergo a physical transformation that forms gel-like structures as a result of interaction between hydrocolloid molecules and water molecules; creating networks that give this material its signature properties.

Hydrocolloids’ gel-like structure has proven beneficial across industries, particularly the food industry. Hydrocolloids can serve as thickeners, stabilizers, emulsifiers, and gelling agents that improve texture, mouthfeel, stability, and appearance in various food products – for instance, pectin is commonly used to give jams and jellies their signature gelled consistency.
Hydrocolloids have become an essential element in medical and pharmaceutical fields for use as wound care products such as hydrocolloid dressings. When applied directly to wounds, hydrocolloid dressings absorb exudate from wounds while creating a moist environment conducive to healing and wound care.
Hydrocolloid dressings possess adhesive qualities, enabling them to adhere tightly to the skin while offering protection from contaminants while creating an ideal wound environment. Hydrocolloid dressings can be commonly used for managing chronic wounds, pressure ulcers, and surgical wounds.
Hydrocolloids possess multiple applications across industries such as food, pharmaceuticals, and wound care. With the ability to form gels or thicken in water-rich environments, hydrocolloids make an effective tool for creating textures with specific textures desired, moisture management needs, and wound healing promotion.
What is the difference between Hydrogel and Hydrocolloid?
The main differences between hydrogel and hydrocolloid can be summarized as follows:
Composition and Structure:
- Hydrogel: Hydrogels are primarily composed of water, with a three-dimensional network structure. They can be made from natural or synthetic materials, such as polysaccharides or proteins.
- Hydrocolloids: Hydrocolloids are substances that can form gels or thicken in the presence of water. They are typically derived from natural sources, including polysaccharides (e.g., pectin, agar, alginate) or proteins (e.g., gelatin, collagen).
Water Absorption and Retention:
- Hydrogel: Hydrogels have a high water absorption capacity and can retain large amounts of water or biological fluids within their network structure without dissolution.
- Hydrocolloid: Hydrocolloids can absorb water and form a gel-like structure. They can absorb moisture from their surroundings, such as wound exudate, and retain it within the gel.
Applications:
- Hydrogel: Hydrogels are widely used in biomedical engineering, tissue engineering, drug delivery systems, contact lenses, and bio-sensing devices.
- Hydrocolloid: Hydrocolloids are commonly used in wound care products, such as hydrocolloid dressings, ostomy care products, dental applications, and as thickeners/stabilizers in the food industry.
Adhesive Properties:
- Hydrogel: Hydrogels typically do not possess strong adhesive properties and may require additional adhesives or methods for secure attachment.
- Hydrocolloid: Hydrocolloids often have inherent adhesive properties, allowing them to adhere to the skin or wound surface without the need for additional adhesives. This property is advantageous in wound dressings and ostomy care products.
Mechanical Properties:
- Hydrogel: Hydrogels can vary in their mechanical properties, ranging from soft and elastic to more rigid and stiff, depending on their composition and crosslinking density.
- Hydrocolloids: Hydrocolloids generally have lower mechanical strength and are more flexible compared to hydrogels.
Both hydrogel and hydrocolloid materials share similar abilities for reacting with water to create gels; however, their composition, water absorption capacities, adhesive properties, mechanical properties, and applications differ considerably.
Hydrogels are widely recognized for their water absorption capabilities and biocompatibility; their applications range from biomedical applications to wound care products and food thickeners. Meanwhile, hydrocolloids often find application as wound care agents or thickening agents within food industry applications.
Hydrogel vs Hydrocolloid in Tabular Form
Here’s a comparison of hydrogel and hydrocolloid materials in a tabular form:
| Properties | Hydrogel | Hydrocolloid |
|---|---|---|
| Composition | Primarily composed of water | Derived from natural sources |
| Water Absorption | High water absorption capacity | Can absorb and retain moisture |
| Adhesive Properties | Generally requires additional adhesives | Often have inherent adhesive properties |
| Mechanical Properties | Can vary from soft to rigid | More flexible and less rigid |
| Applications | Tissue engineering, drug delivery, contact lenses, biosensors, 3D bioprinting | Wound care, ostomy care, dental applications, food industry |
| Swelling Capacity | Swell significantly upon water absorption | Can absorb and swell to a certain extent |
| Biocompatibility | Many hydrogels are biocompatible | Biocompatibility may vary |
Please be aware that this table provides only an overall summary of the properties and applications of hydrogel and hydrocolloid materials. There may be variations and specific characteristics within each category depending on the specific material and formulation used.
Properties and Functionality
Hydrogel and hydrocolloid materials can be defined in three ways. Here is their composition:
Hydrogel:
Properties:
- Swelling Capacity: Hydrogels can absorb and retain large amounts of water or biological fluids within their three-dimensional network structure, leading to swelling.
- Water Content: Hydrogels are composed primarily of water, typically containing 90% to 99% water.
- Biocompatibility: Hydrogel materials often show biocompatibility, meaning that living tissues tolerate them well and they may be used in various biomedical applications. Bio-sensing and Diagnostic Devices: Hydrogels can be customized to detect specific biomarkers for biosensing and diagnostic uses.
- Mechanical Properties: The mechanical properties of hydrogels can be tuned, ranging from soft and elastic to more rigid and stiff, depending on the composition and crosslinking density.
- Biomimetic Characteristics: Hydrogels can mimic the water-rich environment and properties of biological tissues, making them suitable for applications requiring biomimicry.
Functionality:
- Hydrogels’ Water Absorbing and Retaining Capabilities: Hydrogels have the unique capability of absorbing and retaining fluids like blood or bodily fluids, making them suitable for applications like wound dressings or drug delivery systems.
- Drug Release and Delivery: Hydrogels can be utilized as carriers for controlled and targeted drug delivery, allowing for the sustained release of therapeutic agents.
- Tissue Engineering: Hydrogels are widely used as scaffolds for tissue engineering, providing a supportive matrix for cell growth and regeneration.
- Contact Lenses: The high water content and biocompatibility of hydrogels make them suitable for manufacturing soft contact lenses.
- Bio-sensing and Diagnostic Devices: Hydrogels can be functionalized to detect and respond to specific biomarkers, enabling their use in biosensing and diagnostic applications.
Hydrocolloid:
Properties:
- Gel-Forming Ability: Hydrocolloids can form gels or thicken when in contact with water or moisture.
- Water Absorption: Hydrocolloids can absorb water from their surroundings and retain it within their gel-like structure.
- Adhesive Properties: Many hydrocolloids possess natural adhesive qualities that allow them to adhere directly to skin or wound surfaces without additional adhesives being needed for adhesion.
- Flexibility: Hydrocolloids generally exhibit flexibility and conformability, providing comfort and ease of use.
Functionality:
- Moisture Management: Hydrocolloids help manage moisture by absorbing and retaining fluids, creating a moist environment that promotes wound healing and prevents tissue desiccation.
- Exudate Management: Hydrocolloid dressings are effective in managing wound exudate by absorbing it into the dressing, reducing the risk of maceration, and providing a conducive environment for healing.
- Protection Against Contamination: Hydrocolloid dressings create a barrier that protects wounds from external contaminants and bacteria, reducing the risk of infection.
- Adhesion and Wound Coverage: Hydrocolloid dressings adhere to the skin, providing a secure and comfortable covering for wounds.
- Ostomy Care: Hydrocolloid-based ostomy appliances create a seal around stoma sites, preventing leakage and promoting comfort.
Hydrogels and hydrocolloids have different properties and functions. Hydrogels are characterized by their water absorption capabilities, biocompatibility, and versatility, while hydrocolloids are renowned for their moisture management, adhesion, and wound care.
Applications
Applications of hydrogel and hydrocolloid materials include:
Hydrogel:
- Tissue Engineering: Hydrogels are used as scaffolds in tissue engineering. They provide a matrix that supports cell growth and regeneration. They are used in the development of artificial tissues and organs.
- Drug Delivery Systems: Hydrogels are utilized as carriers for controlled and targeted drug delivery. They can encapsulate drugs and release them slowly over time, improving therapeutic efficacy and reducing side effects.
- Contact Lenses: Soft contact lenses are often made from hydrogel materials due to their high water content and biocompatibility. Hydrogels provide comfort and allow oxygen to pass through to the cornea.
- Wound Healing: Hydrogel dressings are used in wound care to create a moist environment that promotes healing. They help manage wound exudate, prevent infection, and protect the wound.
- Biosensors and Diagnostics: Hydrogels can be functionalized with bioactive molecules to detect and respond to specific substances or biomarkers, enabling their use in biosensing and diagnostic devices.
- 3D Bioprinting: Hydrogels are used as bioinks in 3D bioprinting to create complex structures and tissues with high precision.
Hydrocolloid:
- Wound Care: Hydrocolloid dressings are commonly used for the management of various wounds, including pressure ulcers, diabetic ulcers, surgical wounds, and burns. They provide moisture management, protect against contamination, and support wound healing.
- Ostomy Care: Hydrocolloid-based ostomy appliances are used to create a seal around the stoma site, preventing leakage and protecting the peristomal skin. They provide comfort and security for individuals with colostomies or ileostomies.
- Dental Applications: Hydrocolloid materials are used in dentistry for impression taking, making dental molds, and creating dental prostheses. They provide accurate and detailed impressions.
- Food Industry: Food products use hydrocolloids as gelling agents, thickeners, and stabilizers. They improve texture, provide stability, and enhance sensory attributes.
- Pharmaceuticals: Hydrocolloids can be utilized in the formulation of oral dosage forms like tablets and capsules to optimize drug release profiles, increase bioavailability and foster patient compliance.
Here are a few applications of hydrogels and hydrocolloids. They possess unique properties and functions which make them invaluable tools in industries as diverse as biomedical engineering, wound care, dentistry, food, and pharmaceuticals.
Comparison and Contrast
Comparison and contrast between hydrogel and hydrocolloid materials can be summarized as follows:
- Composition:
-
- Hydrogel: Hydrogels are primarily composed of water, with a three-dimensional network structure. They can be made from natural or synthetic materials.
- Hydrocolloids: Hydrocolloids are derived from natural sources such as polysaccharides or proteins, and they can form gels or thicken in the presence of water.
- Water Absorption:
-
- Hydrogel: Hydrogels have a high water absorption capacity and can retain large amounts of water or biological fluids within their network structure without dissolution.
- Hydrocolloid: Hydrocolloids can absorb water and form a gel-like structure, effectively retaining moisture within the gel.
- Adhesive Properties:
-
- Hydrogel: Hydrogels typically do not possess strong adhesive properties and may require additional adhesives or methods for secure attachment.
- Hydrocolloid: Hydrocolloids often have inherent adhesive properties, allowing them to adhere to the skin or wound surface without the need for additional adhesives. This property is advantageous in wound dressings and ostomy care products.
- Mechanical Properties:
-
- Hydrogel: Hydrogels can vary in their mechanical properties, ranging from soft and elastic to more rigid and stiff, depending on the composition and crosslinking density.
- Hydrocolloids: Hydrocolloids generally have lower mechanical strength and are more flexible compared to hydrogels.
- Applications:
-
- Hydrogel: Hydrogels find applications in tissue engineering, drug delivery systems, contact lenses, biosensors, and 3D bioprinting, among others.
- Hydrocolloid: Hydrocolloids are commonly used in wound care products, ostomy care, dental applications, and as thickeners in the food industry.
- Swelling Capacity:
-
- Hydrogel: Hydrogels have a significant swelling capacity due to their ability to absorb and retain water within their network structure.
- Hydrocolloid: Hydrocolloids can absorb water and swell to a certain extent, but their swelling capacity is generally lower compared to hydrogels.
- Biocompatibility:
-
- Hydrogel: Many hydrogel materials exhibit biocompatibility, making them well-tolerated by living tissues and suitable for biomedical applications.
- Hydrocolloids: While hydrocolloids derived from natural sources can be biocompatible, their biocompatibility may vary depending on the specific material used.
Hydrogel and hydrocolloid materials differ in composition, water absorption, adhesive properties, mechanical properties, applications, swelling capacity, and biocompatibility.
Hydrogels are primarily water-based materials with a wide range of applications in tissue engineering and drug delivery, while hydrocolloids are derived from natural sources and find extensive use in wound care and food industry applications.
Conclusion
In conclusion, hydrogel and hydrocolloid materials are distinct but versatile substances with unique properties and applications. Hydrogels are composed primarily of water. They have a large water absorption capability, which makes them ideal for tissue engineering, drug distribution, contact lenses, and biosensors. Hydrocolloids, on the other hand, are derived from sources natural and can thicken or gel in the presence of liquid.
They find applications in wound care, ostomy care, dental applications, and as thickeners in the food industry. While hydrogels have higher mechanical strength and can vary in their elasticity, hydrocolloids are generally more flexible. Additionally, hydrocolloids often have inherent adhesive properties, whereas hydrogels may require additional adhesives for secure attachment.