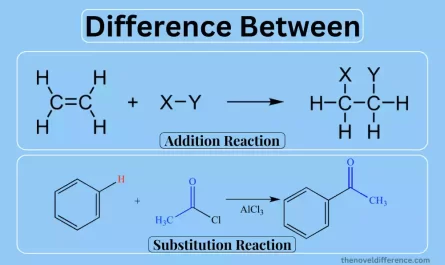
The key difference between PP and PET is PP, which stands for polypropylene, plays a leading role. It is a saturated polymer, a term that signifies a certain stability and resilience. PP is known for its durability, making it a favored choice in a variety of applications. It’s like the steadfast anchor in the polymer world, offering strength, chemical resistance, and reliability. Whether it’s in packaging, automotive components, or textiles, PP shines as a dependable player.
On the other side of the polymer stage is PET, short for polyethylene terephthalate. PET, in contrast to PP, is an unsaturated polymer, giving it a different flavor in this polymer narrative. It is renowned for its transparency and lightweight nature, making it the star in applications that require a clear view or where weight reduction is essential. PET’s versatility is evident in its use in beverage bottles, food containers, textiles, and even the world of electronics.
In this world of polymers, PP and PET represent two distinct characters with their own unique traits and applications. Together, they enrich the polymer story, showing us that there’s a perfect polymer for every role in the grand production of materials and products.
Importance of PP and PET
Understanding the difference between PP and PET is important for several reasons:
1. Material selection: Knowing the distinctions between PP and PET allows for informed material selection for specific applications. Each Material has Unique Properties That Make it Suitable for Different Uses. Selecting the appropriate material ensures optimal performance and functionality.
2. Performance and Durability: PP and PET exhibit different mechanical, thermal, and chemical properties. Understanding these variances helps in choosing the material that can withstand the required conditions, such as temperature, pressure, or chemical exposure. This ensures the durability and longevity of the product or packaging.
3. Environmental impact: PP and PET have different environmental implications. PET is widely recyclable and can be used to produce new PET products, reducing waste and conserving resources. On the other hand, PP recycling rates are lower, and it may have limited recycling options depending on its specific composition. Understanding the environmental impact of each material aids in making sustainable choices.
4. Cost Considerations: PP and PET may differ in terms of production costs, availability, and market pricing. Understanding These Cost Factors can Help in Determining the Most Cost-Effective Option for a Particular Application, Considering Factors Such as Production Volume, Material Availability, and Budget Constraints.
5. Safety and Regulatory Compliance: Different industries and applications may have specific safety and regulatory requirements. Understanding the properties and characteristics of PP and PET helps ensure compliance with applicable regulations, standards, and certifications.
6. Consumer Perception and Preference: Consumer perception and preference play a significant role in product packaging and branding. Understanding the difference between PP and PET enables companies to make informed decisions regarding packaging materials, considering factors such as aesthetics, clarity, and consumer expectations.
Understanding the difference between PP and PET facilitates informed decision-making regarding material selection, performance requirements, environmental impact, cost considerations, safety compliance, and consumer preferences. This knowledge ensures that the chosen material aligns with the specific needs and goals of the application or product.
What is Polypropylene (PP)?
Polypropylene (PP) is a type of thermoplastic polymer that is widely used in various applications due to its versatile properties. It is a member of the polyolefin family of polymers and is known for its combination of strength, rigidity, and chemical resistance.
Here are some key characteristics and uses of Polypropylene:
Key Characteristics of Polypropylene (PP):
- High Melting Point: PP has a relatively high melting point, which makes it suitable for applications where heat resistance is important.
- Chemical Resistance: It is highly resistant to many chemicals, acids, and bases, making it an ideal choice for containers and packaging in the chemical and pharmaceutical industries.
- Durable and Tough: PP is known for its durability and toughness. It can withstand mechanical stress and impact, which makes it suitable for applications where structural integrity is crucial.
- Lightweight: Despite its strength, PP is a lightweight material, which is advantageous in products where weight is a consideration, such as automotive components and packaging.
- Low Moisture Absorption: PP has low moisture absorption, making it suitable for applications that require resistance to moisture and water.
- Electrical Insulation: It is an excellent electrical insulator, making it valuable in electrical and electronic applications.
Common Applications of Polypropylene (PP):
- Packaging: PP is extensively used in the packaging industry for products such as bottles, caps, containers, and food packaging.
- Automotive: It is used in the automotive industry for various components, including bumpers, interior trim, and battery cases.
- Furniture: PP is used in the production of lightweight, durable, and weather-resistant outdoor furniture.
- Textiles: It is used in textiles for applications such as ropes, geotextiles, and woven bags.
- Medical Devices: In the medical field, PP is used for disposable syringes, test tubes, and other medical devices.
- Industrial Applications: PP is used in various industrial applications, including pipes, fittings, and sheet materials.
- Stationery: It is used in stationery items like folders, binders, and packaging materials.

Polypropylene is a highly versatile and cost-effective polymer that finds use in a wide range of industries due to its unique combination of properties and its ability to be easily processed through methods such as injection molding, extrusion, and blow molding.
What is Polyethylene Terephthalate (PET)?
Polyethylene Terephthalate (PET) is a versatile thermoplastic polymer known for its clarity, lightweight nature, and excellent mechanical properties. It is a type of polyester and is commonly used in various applications, especially in the packaging industry.
Here are the key characteristics and uses of Polyethylene Terephthalate (PET):
Key Characteristics of Polyethylene Terephthalate (PET):
- Transparency: PET is a transparent polymer, making it ideal for applications where visibility or showcasing of contents is important, such as beverage containers.
- Lightweight: It is a lightweight material, which is advantageous for applications where weight reduction is essential, like in the production of beverage bottles and food containers.
- Strength and Durability: PET offers good mechanical properties, including strength, impact resistance, and toughness, making it suitable for a wide range of applications.
- Chemical Resistance: While not as chemically resistant as Polypropylene (PP), PET is still resistant to many common chemicals, acids, and bases.
- Heat Resistance: PET has moderate heat resistance and can withstand temperatures up to a certain threshold, making it suitable for heat-setting processes during manufacturing.
- Recyclability: PET is highly recyclable, and recycled PET, known as rPET, is commonly used in the production of new PET products, contributing to its sustainability.
Common Applications of Polyethylene Terephthalate (PET):
- Beverage Bottles: PET is widely used in the production of beverage bottles for carbonated drinks, water, juices, and other liquids due to its transparency and lightweight properties.
- Food Packaging: It is commonly used in food packaging for items like jars, trays, and containers, especially for products that benefit from being showcased through transparent packaging.
- Textiles: PET is used in the textile industry to produce synthetic fibers used in clothing, upholstery, and carpeting. These fibers are known as polyester fibers.
- Electronics: PET films are used in electronic devices as insulating materials, displays, and touchscreens.
- Medical Devices: In the medical field, PET is used in various applications, including disposable medical containers and imaging technologies like PET scans (Positron Emission Tomography).
- Thermoforming: PET sheets can be thermoformed into various shapes and are used in applications such as blister packaging and food containers.
- Engineering Plastics: In some cases, PET is reinforced with glass fibers to create engineering plastics used in automotive components and industrial parts.
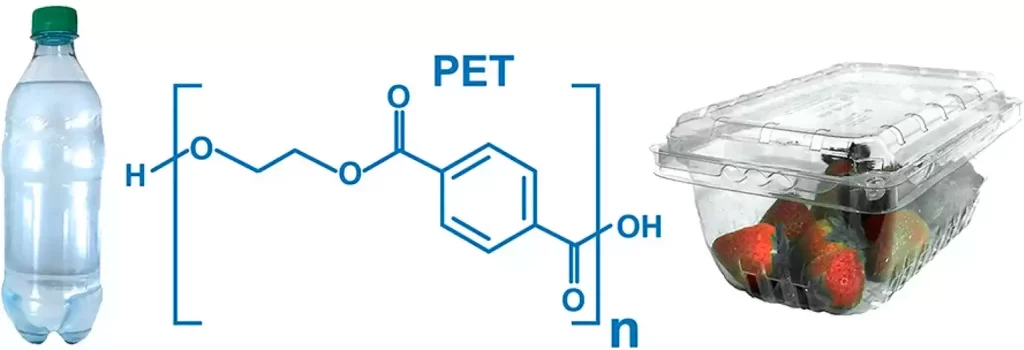
Polyethylene Terephthalate (PET) is a widely used polymer due to its unique combination of properties, including transparency, strength, and recyclability. Its applications range from everyday beverage bottles to specialized uses in various industries.
What is the Difference Between PP and PET?
The Main Differences Between PP (Polypropylene) and PET (Polyethylene Terephthalate) can be summarized as follows:
1. Chemical Composition: PP Is a Thermoplastic Polymer Derived From Propylene Monomers, While PET Is a Thermoplastic Polyester Made From Ethylene Glycol and Terephthalic Acid. The different chemical compositions result in variations in their properties and applications.
2. Density: PP has a lower density than PET. PP Typically Has a Density Ranging From 0.895 to 0.92 G/cm³, While PET Has a Higher Density Ranging From 1.34 to 1.40 G/cm³. This difference in density affects their weight, with PP being lighter than PET.
3. Transparency: PP is generally translucent, while PET is highly transparent. PET offers excellent clarity and visibility, making it suitable for applications where the visibility of contents is important, such as beverage bottles. PP can be made opaque with the addition of pigments or fillers.
4. Melting Point: PP has a lower melting point compared to PET. The melting point of PP is around 160-170°C (320-338°F), while the melting point of PET is around 245-255°C (473-491°F). The difference in melting points affects the processing and manufacturing techniques used for each material.
5. Mechanical Properties: PP and PET have different mechanical properties. PP has good tensile strength, flexibility, and impact resistance. It is known for its toughness and ability to withstand repeated bending or deformation. PET, on the other hand, has high tensile strength, stiffness, and excellent impact resistance. It offers rigidity and dimensional stability.
6. Chemical Resistance: PP exhibits high chemical resistance, making it resistant to many acids, alkalis, and organic solvents. PET also has good chemical resistance but may be more susceptible to certain solvents and chemicals compared to PP.
7. Environmental Sustainability: PET is widely recycled and can be reused to produce new PET products. It is commonly used in recycling programs. PP, although recyclable, has a lower recycling rate and may have limited recycling options depending on its specific composition.
8. Applications: PP is commonly used in packaging, automotive parts, textiles, medical devices, and various consumer products. PET is widely used for beverage bottles, food packaging, textiles, electrical components, and engineering materials.
Understanding the differences between PP and PET is important for selecting the appropriate material for specific applications, considering factors such as mechanical properties, chemical resistance, transparency, and recycling options.
Advantages and disadvantages of PP and PET
Advantages of Polypropylene (PP):
- Chemical Resistance: PP is highly resistant to a wide range of chemicals, acids, and bases. This property makes it ideal for applications involving exposure to various chemical solvents.
- Durability: PP is known for its toughness and durability. It can withstand mechanical stress, making it suitable for applications requiring strength and impact resistance.
- Heat Resistance: It has a high melting point, which makes it suitable for hot-fill applications, including the packaging of hot liquids and food items.
- Low Moisture Absorption: PP has low moisture absorption, making it a suitable choice for applications that require resistance to moisture and water.
- Lightweight: Despite its strength, PP is a lightweight material. This is advantageous in products where weight reduction is a consideration, such as automotive components and packaging.
- Electrical Insulation: It is an excellent electrical insulator, making it valuable in electrical and electronic applications.
Disadvantages of Polypropylene (PP):
- Opaque: PP is not as transparent as some other plastics like PET, which limits its use in applications requiring high transparency.
- Environmental Impact: While it is recyclable, the recycling rate for PP is lower than that of PET. This can impact its sustainability and environmental footprint.
Advantages of Polyethylene Terephthalate (PET):
- Transparency: PET is transparent and offers excellent clarity, making it ideal for applications where visibility or showcasing of contents is essential.
- Lightweight: It is a lightweight material, which is advantageous for applications where weight reduction is important, such as in beverage bottles.
- Strength and Durability: PET offers good mechanical properties, including strength and toughness, making it suitable for a wide range of applications.
- Recyclability: PET is highly recyclable, and recycled PET (rPET) is commonly used in the production of new PET products, contributing to its sustainability.
Disadvantages of Polyethylene Terephthalate (PET):
- Heat Resistance: PET has moderate heat resistance, and it may not be suitable for very high-temperature applications. It can deform at high temperatures.
- Chemical Resistance: While it is resistant to common chemicals, it is not as chemically resistant as PP.
- Limited Chemical Resistance: PET may not be suitable for applications that require resistance to a wide range of chemicals and solvents, as it has limitations in this regard.
- Recycling Challenges: PET can present challenges in recycling, especially when dealing with colored or contaminated plastics, which can affect its purity in the recycling process.
The advantages and disadvantages of PP and PET determine their suitability for various applications in different industries, and understanding these characteristics is essential for making informed material choices.
Applications of PP or PET
Polypropylene (PP) and Polyethylene Terephthalate (PET) are used in a wide range of applications across various industries due to their unique properties.
Here are some common applications of both materials:
Applications of Polypropylene (PP):
- Packaging: PP is widely used in packaging applications, including plastic bags, flexible packaging films, and containers. It is a popular choice for food packaging, as it provides a good barrier against moisture and chemicals.
- Automotive Components: PP is used in automotive components such as bumpers, interior trim, dashboard panels, and under-the-hood parts due to its durability and heat resistance.
- Textiles: PP is used to produce synthetic textiles like ropes, geotextiles, and woven bags. It is known for its resistance to abrasion and UV radiation, making it suitable for outdoor fabrics.
- Medical Devices: In the medical field, PP is used for disposable syringes, test tubes, and various medical containers and devices due to its chemical resistance and low toxicity.
- Furniture: PP is used in the production of lightweight, durable, and weather-resistant outdoor furniture, as well as in the manufacturing of chairs and tables.
- Stationery: It is used in stationery items such as folders, binders, and packaging materials due to its stiffness and durability.
Applications of Polyethylene Terephthalate (PET):
- Beverage Bottles: PET is one of the most commonly used materials for beverage bottles, including those for water, carbonated drinks, juices, and other liquids due to its transparency and lightweight properties.
- Food Packaging: It is used extensively in food packaging, including containers, jars, trays, and food-grade films, especially for products that benefit from being showcased through transparent packaging.
- Textiles: PET is used in the textile industry to produce synthetic fibers used in clothing, upholstery, and carpeting. These fibers are known as polyester fibers.
- Electronics: PET films are used in electronic devices as insulating materials, displays, and touchscreens due to their electrical insulation properties.
- Medical Devices: In the medical field, PET is used in various applications, including disposable medical containers and imaging technologies like PET scans (Positron Emission Tomography).
- Thermoforming: PET sheets can be thermoformed into various shapes and are used in applications such as blister packaging and food containers.
- Engineering Plastics: In some cases, PET is reinforced with glass fibers to create engineering plastics used in automotive components and industrial parts.
Both PP and PET have a wide array of applications across industries, and their properties make them suitable for diverse uses in manufacturing, packaging, and more. The choice between the two materials depends on the specific requirements and characteristics of the application.
Conclusion
Understanding the difference between PP and PET is essential for selecting the right material for your specific needs. While both materials have their advantages and applications, PP offers superior chemical resistance and flexibility, while PET excels in transparency and impact resistance. By considering their unique properties, you can make informed decisions regarding their usage in various industries, from packaging to automotive and textiles.
So, Whether You’re a Manufacturer, a Consumer, or Anyone Interested in the World of Plastics, Knowing the Difference Between PP and PET Empowers You to Make Choices That Align With Your Requirements and Priorities.