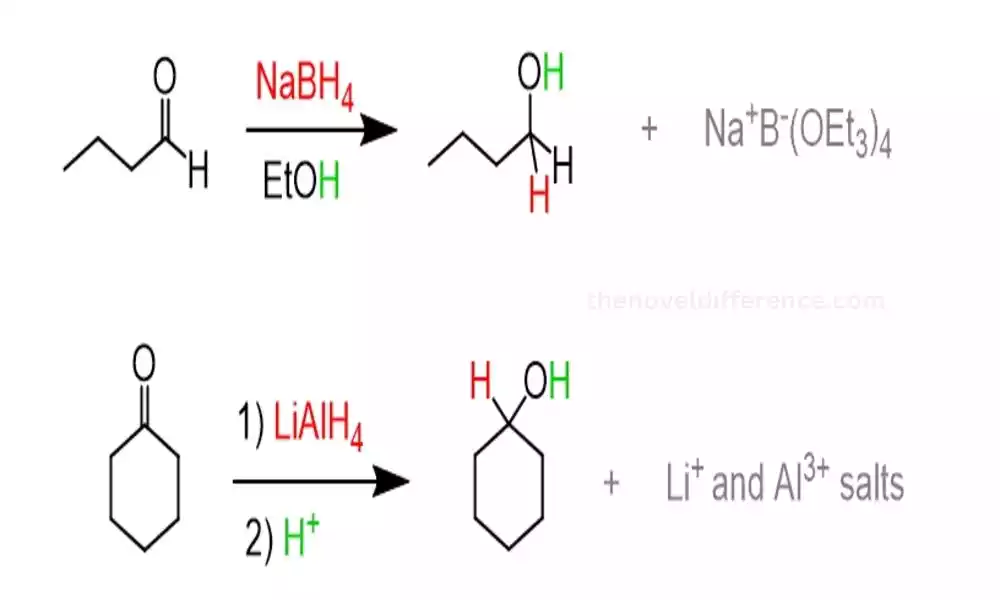
A brief explanation of LiAlH4 and NaBH4
LiAlH4 (Lithium Aluminum Hydride) and NaBH4 (Sodium Borohydride) are both commonly used reducing agents in organic chemistry. They are used to selectively reduce various functional groups, such as carbonyl compounds, in organic synthesis.
LiAlH4 (Lithium Aluminum Hydride): LiAlH4 is a powerful reducing agent with the chemical formula LiAlH4. It is a white crystalline solid that is highly reactive and sensitive to moisture and air. LiAlH4 can effectively reduce an array of functional groups, including ketones, aldehydes, esters, and carboxylic acids. amides, in their corresponding alcohols, or amines.
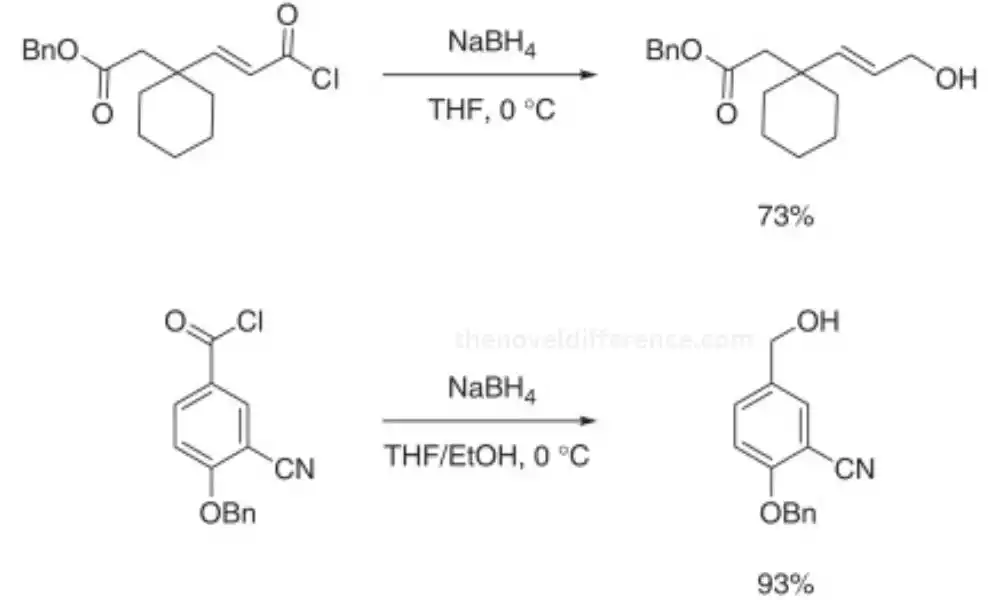
NaBH4 (Sodium Borohydride): NaBH4, on the other hand, is a milder reducing agent with the chemical formula NaBH4. It is a white crystalline powder that is more stable compared to LiAlH4. NaBH4 is commonly used for the reduction of aldehydes and ketones, and it exhibits high selectivity towards these functional groups. It is less reactive to other carbonyl compounds like esters, carboxylic acids, and amides.
The main difference between LiAlH4 and NaBH4 lies in their reactivity and selectivity. LiAlH4 is a more potent reducing agent and can reduce a broader range of functional groups compared to NaBH4.
However, due to its higher reactivity, LiAlH4 requires more cautious handling and specific reaction conditions. NaBH4, on the other hand, is easier to handle and generally safer to use but has a more limited scope of reactivity.
Understanding the differences between LiAlH4 and NaBH4 is crucial for organic chemists when choosing the appropriate reducing agent for specific reactions and applications.
Factors such as the desired selectivity, reaction conditions, and safety considerations play a significant role in determining the suitability of these reducing agents in different synthetic scenarios.
Importance of these compounds in organic synthesis
LiAlH4 and NaBH4 are both important compounds in organic synthesis due to their ability to act as reducing agents. Reduction reactions are fundamental in organic chemistry as they allow the conversion of various functional groups into more reduced forms.
LiAlH4 and NaBH4 play an indispensable role in organic synthesis; here is their significance in brief:
Selective Reduction: LiAlH4 and NaBH4 can be effectively employed to selectively convert carbonyl compounds, including aldehydes and ketones, into their associated alcohols. This selectivity allows for the controlled modification of functional groups, enabling the synthesis of a wide range of organic compounds.
Versatility: LiAlH4 is a highly versatile reducing agent that can reduce not only carbonyl compounds but also other functional groups such as esters, carboxylic acids, and amides. This versatility makes LiAlH4 a valuable tool in the synthesis of complex organic molecules.
Mild Reduction: NaBH4 is often preferred for its milder reducing properties than LiAlH4. It is particularly useful for reducing aldehydes and ketones without affecting more sensitive functional groups present in the molecule. The mild nature of NaBH4 allows for better control and selectivity in reactions.
Synthetic Efficiency: Reduction reactions using LiAlH4 or NaBH4 can lead to significant improvements in synthetic efficiency. Converting carbonyl compounds to alcohols is an integral step in producing pharmaceuticals, natural products, and fine chemicals. By utilizing these reducing agents, chemists can achieve the desired transformations more efficiently and with higher yields.
Compatibility with Other Functional Groups: LiAlH4 and NaBH4 generally exhibit good compatibility with other functional groups commonly found in organic molecules. This compatibility allows for the reduction of carbonyl groups in the presence of other sensitive functional groups without causing undesired side reactions or damage.
Wide Availability: Both LiAlH4 and NaBH4 are commercially available and relatively affordable. Their widespread availability makes them accessible to researchers and enables their frequent use in various organic synthesis laboratories.
LiAlH4 and NaBH4 play crucial roles in organic synthesis as effective and selective reducing agents. Their versatility, mildness, compatibility with other functional groups, and wide availability make them indispensable tools for chemists in developing new molecules and synthesizing complex organic compounds.
Overview of their chemical properties and reactivity
LiAlH4 (Lithium Aluminum Hydride) and NaBH4 (Sodium Borohydride) possess distinct chemical properties and reactivity due to their structural and electronic differences.
Here’s an overview of their chemical properties and reactivity:
LiAlH4:
Chemical Structure: LiAlH4 consists of a lithium cation (Li+) and a tetrahedral AlH4- anion.
Reactivity: LiAlH4 is a strong reducing agent. It reacts vigorously with protic solvents, such as water and alcohol, liberating hydrogen gas. As it exhibits high chemical reactivity with many functional groups – carbonyl compounds (aldehydes, ketones, esters, carboxylic acids, and amides), epoxides, and some halides among them – it makes an attractive candidate for use as an intermediate chemical precursor in production processes.
Reduction Capability: LiAlH4 can reduce carbonyl compounds to their corresponding alcohols or amines. The reduction proceeds via a nucleophilic addition of the hydride (H-) ion to the electrophilic carbon of the carbonyl group, followed by protonation. It is capable of reducing multiple carbonyl groups in a molecule, making it valuable for complex organic synthesis.
Sensitivity: LiAlH4 is highly sensitive to moisture and air, necessitating careful handling. It should be stored under an inert atmosphere and dissolved in dry, aprotic solvents before use.
NaBH4:
Chemical Structure: NaBH4 consists of a sodium cation (Na+) and a tetrahedral BH4- anion.
Reactivity: NaBH4 is a milder reducing agent compared to LiAlH4. It is more stable and less reactive towards protic solvents. However, it still reacts with water slowly. NaBH4 is primarily used for the reduction of carbonyl compounds, specifically aldehydes, and ketones.
Reduction Capability: NaBH4 reduces carbonyl compounds by donating a hydride ion (H-) to the electrophilic carbon of the carbonyl group. This reduction occurs via a nucleophilic addition followed by protonation. NaBH4 is selective towards aldehydes and ketones and does not readily reduce esters, carboxylic acids, or amides under typical reaction conditions.
NaBH4: NaBH4 is more stable in aqueous solutions compared to LiAlH4. It can be dissolved in water to form a basic solution, making it suitable for certain reduction reactions performed in aqueous media.
Both LiAlH4 and NaBH4 have distinct reactivity profiles, making them useful for different synthetic applications. LiAlH4’s strong reducing power and broad scope of reactivity allow for more diverse transformations, while NaBH4’s milder nature and selectivity towards aldehydes and ketones provide better control in specific reduction reactions.
Understanding their reactivity helps chemists choose the appropriate reducing agent for their desired transformations in organic synthesis.
LiAlH4 (Lithium Aluminum Hydride)
LiAlH4 (Lithium Aluminum Hydride) is a powerful reducing agent commonly used in organic synthesis.
Here’s a detailed overview of LiAlH4:
Chemical Structure and Formula:
LiAlH4 consists of a lithium cation (Li+) and a tetrahedral AlH4- anion.
Its chemical formula is LiAlH4, indicating one lithium atom, one aluminum atom, and four hydrogen atoms.
Physical Properties:
LiAlH4 is a white crystalline solid.
It has a high melting point of around 150°C.
The compound is insoluble in nonpolar solvents but reacts with protic solvents, such as water and alcohol.
Preparation Methods:
LiAlH4 is typically prepared by the reaction of lithium hydride (LiH) with aluminum chloride (AlCl3) in anhydrous diethyl ether as the solvent.
The reaction can be represented as LiH + AlCl3 → LiAlH4 + LiCl
Reactivity and Reduction Capabilities:
LiAlH4 is a strong reducing agent, capable of reducing a wide range of functional groups.
It readily donates a hydride ion (H-) to react with electrophilic carbons in various organic compounds.
Carbonyl compounds like aldehydes, ketones, esters, carboxylic acids, and amides should be broken down to their alcohol or amine equivalent for safe disposal.
The reduction proceeds via a nucleophilic addition of the hydride ion to the electrophilic carbon of the carbonyl group, followed by protonation.
Limitations and Safety Considerations:
LiAlH4 is highly sensitive to moisture and air. It reacts vigorously with water or even traces of moisture, releasing flammable hydrogen gas. Therefore, it should be handled and stored under an inert atmosphere.
It requires anhydrous conditions and dry, aprotic solvents for reactions to proceed smoothly.
The reaction with LiAlH4 can be exothermic and potentially hazardous if not controlled properly.
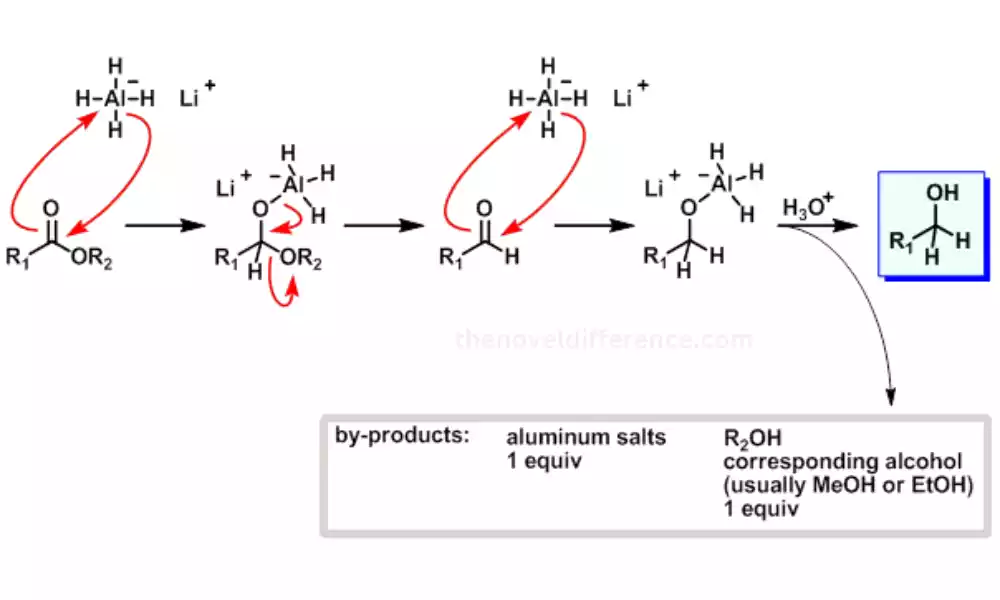
LiAlH4’s strong reducing power and broad reactivity make it a valuable tool in organic synthesis for the conversion of various functional groups. However, its sensitivity to moisture and air requires careful handling and specific reaction conditions to ensure safety and successful reactions.
Chemical structure and formula
The chemical structure of LiAlH4 (Lithium Aluminum Hydride) consists of a lithium cation (Li+) and a tetrahedral AlH4- anion. The formula for LiAlH4 is LiAlH4, indicating one lithium atom (Li), one aluminum atom (Al), and four hydrogen atoms (H).
The aluminum atom is surrounded by four hydrogen atoms in a tetrahedral arrangement, forming the AlH4- anion. The lithium cation is associated with the AlH4- anion to maintain charge neutrality in the compound. Overall, LiAlH4 is a salt-like compound with an ionic structure.
NaBH4 (Sodium Borohydride)
NaBH4 (Sodium Borohydride) is a commonly used reducing agent in organic chemistry.
Here’s an overview of NaBH4:
Chemical Structure and Formula:
NaBH4 consists of a sodium cation (Na+) and a tetrahedral BH4- anion.
NaBH4 can be represented as one sodium (Na), one boron (B), and four hydrogens (H). These four bonds connect them.
The boron atom is tetrahedrally coordinated with the four hydrogen atoms.
Physical Properties:
NaBH4 is a white crystalline solid.
It has a relatively high melting point of around 400°C.
The compound is sparingly soluble in many organic solvents but readily dissolves in water.
Preparation Methods:
NaBH4 is typically prepared by the reaction of sodium hydride (NaH) with trimethyl borate (B(OCH3)3) or boron trichloride (BCl3).
The reaction can be represented as: NaH + B(OCH3)3 → NaBH4 + 3CH3OH
Reactivity and Reduction Capabilities:
NaBH4 is a mild reducing agent.
It readily donates a hydride ion (H-) to react with electron-deficient species, such as carbonyl compounds (aldehydes and ketones).
The reduction proceeds via a nucleophilic addition of the hydride ion to the electrophilic carbon of the carbonyl group, followed by protonation.
Limitations and Safety Considerations:
NaBH4 is generally considered safe to handle and use compared to more reactive reducing agents.
However, it should be stored in a dry environment to prevent degradation and moisture absorption.
Although NaBH4 is less reactive compared to LiAlH4, it can still react with water, acids, and some oxidizing agents, producing flammable hydrogen gas.
Applications:
NaBH4 is widely used for the selective reduction of aldehydes and ketones to their corresponding alcohols in organic synthesis.
It is commonly employed in the pharmaceutical industry for the synthesis of various drug intermediates and active pharmaceutical ingredients (APIs).
NaBH4 also finds applications in the production of fine chemicals, and polymers, and as a reducing agent in certain industrial processes.
NaBH4 offers a milder and more selective reduction capability compared to stronger reducing agents like LiAlH4. Its relative ease of handling and availability make it a popular choice for many reduction reactions in organic synthesis.
Chemical structure and formula
The chemical structure of NaBH4 (Sodium Borohydride) can be represented as follows:
|
H — B — H
|
Na
NaBH4 (Na-Boron-4 Hydroxides) can be represented in chemical form as the combination of four sodium (Na) atoms bound together with one boron (B), as well as four hydrogen (H).
The boron atom is tetrahedrally coordinated to the four hydrogen atoms, with a sodium atom associated with the BH4- anion to maintain charge neutrality in the compound.
NaBH4 is an ionic compound consisting of a sodium cation (Na+) and a borohydride anion (BH4-). The borohydride anion is negatively charged, with the boron atom bearing a negative charge (-1) and each hydrogen atom attached to it.
Comparison between LiAlH4 and NaBH4
When comparing LiAlH4 (Lithium Aluminum Hydride) and NaBH4 (Sodium Borohydride), several key differences emerge in terms of their reducing power, selectivity, reaction conditions, applications, and availability.
Here’s a comparison between the two compounds:
Reducing Power:
LiAlH4: LiAlH4 is a stronger reducing agent compared to NaBH4. Carbonyl compounds, such as ketones, aldehydes as well as carboxylic acids and amides) and also certain epoxides, halides, and epoxides can all be reduced with this method.
NaBH4: NaBH4 is a milder reducing agent compared to LiAlH4. Primarily used to reduce carbonyl compounds – particularly aldehydes and ketones.
Selectivity:
LiAlH4: Due to its strong reducing power, LiAlH4 exhibits less selectivity towards specific functional groups. It can reduce various carbonyl compounds and other functional groups, allowing for more diverse transformations.
NaBH4: NaBH4 is more selective in its reduction capabilities. It predominantly reduces aldehydes and ketones, while showing lower reactivity towards esters, carboxylic acids, and amides.
Reaction Conditions:
LiAlH4: LiAlH4 reactions typically require anhydrous conditions and dry, aprotic solvents to prevent unwanted side reactions. It is highly sensitive to moisture and air, necessitating careful handling under an inert atmosphere.
NaBH4: NaBH4 reactions can be performed under milder conditions. It is more stable in aqueous solutions and can be dissolved in water to form a basic solution, making it suitable for certain reduction reactions performed in aqueous media.
Applications:
LiAlH4: LiAlH4 finds extensive use in organic synthesis for the reduction of various functional groups. Its strong reducing power and broad reactivity make it valuable for the synthesis of complex organic molecules.
NaBH4: NaBH4 is commonly employed for the selective reduction of aldehydes and ketones in various organic transformations. It is widely used in the synthesis of pharmaceuticals, fine chemicals, and other organic compounds.
Availability:
LiAlH4: LiAlH4 is commercially available and commonly used in organic synthesis laboratories. However, its sensitivity to moisture and air may require special storage and handling precautions.
NaBH4: NaBH4 is more readily available and easier to handle compared to LiAlH4. It is widely accessible and commonly used in organic chemistry laboratories.
The choice between LiAlH4 and NaBH4 depends on the specific requirements of the reduction reaction, the desired selectivity, and the reaction conditions. LiAlH4 offers greater reducing power and versatility, while NaBH4 provides milder conditions and selectivity towards aldehydes and ketones.
What are the similarities between LiAlH4 and NaBH4?
While LiAlH4 (Lithium Aluminum Hydride) and NaBH4 (Sodium Borohydride) have distinct differences, they also share some similarities:
Reducing Agents: LiAlH4 and NaBH4 are widely utilized reducing agents in organic chemistry. They are capable of transferring hydride ions (H-) to electron-deficient species, facilitating reduction reactions.
Carbonyl Reduction: Both compounds are effective at breaking down carbonyl compounds like aldehydes and ketones into their constituent parts, providing effective carbon reduction solutions. They donate hydride ions to the electrophilic carbon of the carbonyl group, resulting in the formation of alcohols.
Synthetic Applications: LiAlH4 and NaBH4 find extensive applications in organic synthesis. They are utilized for the synthesis of various organic compounds, including pharmaceuticals, fine chemicals, and natural products.
Safety Considerations: Although both compounds require care during handling, they share some safety considerations. LiAlH4 and NaBH4 should be stored in a dry and moisture-free environment, as they are sensitive to moisture and can react violently with water or protic solvents.
Selectivity: While LiAlH4 has broader reactivity, both compounds display selectivity in their reduction capabilities. LiAlH4 exhibits a wider range of reactivity towards functional groups, whereas NaBH4 is more selective towards aldehydes and ketones.
Availability: Both LiAlH4 and NaBH4 are commercially available and widely accessible in the field of organic chemistry. They are commonly found in reagent catalogs and used in research laboratories.
Despite their differences, LiAlH4 and NaBH4 share fundamental characteristics as versatile reducing agents, making them invaluable tools in organic synthesis.
LiAlH4 vs NaBH4 in Tabular Form
Sure! Here’s a comparison between LiAlH4 (Lithium Aluminum Hydride) and NaBH4 (Sodium Borohydride) in a tabular form:
| Aspect | LiAlH4 | NaBH4 |
|---|---|---|
| Chemical Formula | LiAlH4 | NaBH4 |
| Reducing Power | Strong reducing agent | Milder reducing agent |
| Selectivity | Broad reactivity | Selective towards aldehydes and ketones |
| Reactivity towards | Carbonyl compounds, | Aldehydes and ketones |
| functional groups | esters, carboxylic acids, | |
| amides, epoxides, | ||
| and some halides | ||
| Reaction Conditions | Anhydrous conditions, | Milder conditions, more |
| dry, aprotic solvents | stable in aqueous solutions | |
| Handling Sensitivity | Sensitive to moisture | Less sensitive to moisture |
| Applications | Synthesis of complex | Reduction of aldehydes, |
| organic molecules | ketones, and other compounds | |
| Availability | Commercially available | Widely accessible |
Please note that this table provides a general overview, and there may be specific cases or variations in the use of LiAlH4 and NaBH4 depending on the particular reaction or synthetic goals.
Conclusion
LiAlH4 (Lithium Aluminum Hydride) and NaBH4 (Sodium Borohydride) are both important reducing agents in organic synthesis, but they have distinct differences.
LiAlH4 is an effective reducing agent with a broad scope that is capable of reacting with many functional groups including carbonyl compounds, esters, carboxylic acids, and amides. It requires anhydrous conditions and careful handling due to its sensitivity to moisture and air.
On the other hand, NaBH4 is a milder reducing agent, primarily used for the selective reduction of aldehydes and ketones. It is more stable in aqueous solutions and can be dissolved in water, making it suitable for certain reduction reactions performed in aqueous media. NaBH4 is easier to handle and more readily available compared to LiAlH4.