Definition of Schottky Defect and Frenkel Defect
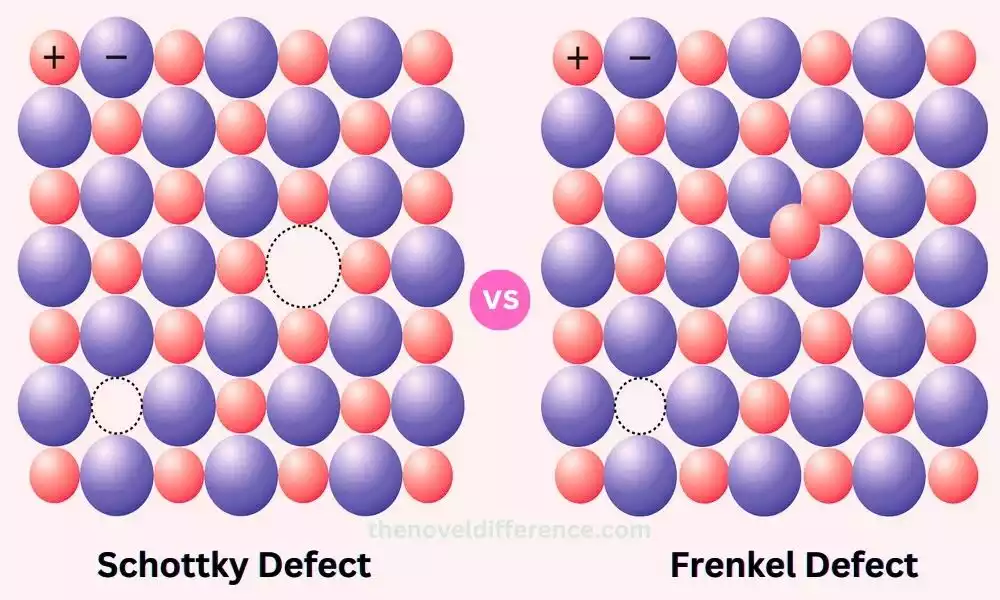
Schottky Defect: Schottky defect is a type of point defect that occurs in ionic crystals. It involves the simultaneous creation of vacancies for both cations and anions within the crystal lattice, resulting in a defect that maintains electrical neutrality. This defect is named after the German physicist Walter H. Schottky, who extensively studied point defects in crystals.
Frenkel Defect: Frenkel defect is another type of point defect that occurs in crystal structures, particularly in ionic crystals. It involves the displacement of an ion from its normal lattice site to an interstitial position within the crystal. The displaced ion remains within the crystal, maintaining electrical neutrality. The Frenkel defect is named after the Russian scientist Yakov Frenkel, who extensively studied point defects in crystals.
Both Schottky and Frenkel defects are point defects, which are localized irregularities or imperfections in the crystal lattice structure. They can occur during crystal growth, under thermal activation, or due to high-energy radiation.
Schottky defects involve the creation of vacancies for both cations and anions, while Frenkel defects involve the displacement of ions to interstitial positions. These defects significantly impact the properties and behavior of materials and thus play a pivotal role in materials science.
Overview of crystal defects
Crystal defects are irregularities or imperfections within a solid material’s crystal lattice structure. They can arise during the formation, growth, or processing of crystals and can have significant effects on the physical, chemical, and mechanical properties of materials. Crystal defects can be divided into various categories, such as point defects, line defects, and planar defects.
Here’s an overview of each type:
Point Defects:
Point defects are localized deviations from the ideal crystal lattice at specific atomic or molecular positions.
They include vacancies, interstitials, substitutional impurities, and self-interstitials.
Vacancies occur when lattice sites are unoccupied, while interstitials are atoms or molecules occupying positions between the lattice sites.
Substitutional impurities involve the replacement of one type of atom with another within the crystal lattice.
Self-interstitials occur when atoms from the crystal move into interstitial positions.
Line Defects (Dislocations):
Dislocations are linear defects in the crystal lattice structure that occur due to misalignments or disruptions in the regular arrangement of atoms.
Edge dislocations result from an extra half-plane of atoms inserted into the crystal lattice, leading to a step-like structure.
Screw dislocations involve a spiral-like distortion of the crystal lattice along the dislocation line.
Dislocations can affect the mechanical properties of materials, such as their strength, plasticity, and deformation behavior.
Planar Defects:
Planar defects occur as two-dimensional irregularities in the crystal lattice structure.
Grain boundaries are interfaces between two adjacent grains with different crystal orientations.
Twin boundaries are particular grain boundaries where mirror-image crystal domains are joined together.
Stacking faults occur when an error occurs in a crystal’s stacking sequence of atomic layers.
Interfaces, such as surfaces or interfaces with amorphous regions, can also be considered planar defects.
Crystal defects can alter various material properties, including electrical conductivity, thermal conductivity, mechanical strength, optical properties, and chemical reactivity.
They can affect diffusion processes, phase transformations, and the behavior of materials under external stimuli such as temperature, pressure, or electromagnetic fields. Understanding and controlling crystal defects is essential in materials science and engineering to tailor material properties for specific applications, optimize performance, and prevent material failure.
Importance of crystal defects in materials science
Crystal defects play an integral part in materials science due to their significant effects on material properties and behavior.
Understanding and controlling crystal defects is essential for several reasons:
- Property Modification: Crystal defects can modify the physical, chemical, mechanical, and electronic properties of materials. By intentionally introducing specific defects, scientists and engineers can tailor material properties to meet specific requirements, such as enhancing conductivity, improving mechanical strength, or optimizing optical properties.
- Materials Design and Development: Crystal defects provide avenues for designing and developing novel materials with improved or unique properties. By manipulating the types, densities, and distributions of defects, researchers can create materials with enhanced functionalities, such as high-temperature superconductors, catalytic materials, or materials with tailored magnetic properties.
- Phase Transformations: Crystal defects influence phase transformations, which are changes in the arrangement or composition of atoms within a material. Defects can affect the kinetics and thermodynamics of phase transformations, leading to changes in microstructure and material behavior. Understanding these effects is crucial for materials processing, such as heat treatment, annealing, or alloying.
- Diffusion and Transport: Crystal defects, particularly point defects, significantly impact diffusion and transport phenomena in materials. Vacancies, interstitials, and dislocations provide pathways for atomic or molecular movement, affecting processes like atomic diffusion, ionic conduction, and mass transport. Defect engineering can enhance or inhibit these processes, enabling the design of materials with tailored transport properties.
- Mechanical Properties: Crystal defects, especially dislocations, strongly influence the mechanical properties of materials. They affect the deformation behavior, strength, hardness, and ductility of materials. By controlling dislocation densities and distributions, researchers can design materials with improved mechanical properties or enhanced resistance to deformation or fracture.
- Material Stability and Degradation: Crystal defects can influence the stability and degradation of materials. They can affect material aging, creep, fatigue, and failure mechanisms. Understanding defect interactions and their effects on material stability is crucial for ensuring the long-term reliability and durability of materials.
- Materials Characterization: Crystal defects provide valuable information for materials characterization techniques. Defects affect the scattering of various probes, such as X-rays, electrons, or photons, providing insights into the crystal structure, composition, and defects themselves. Analyzing defects is central to techniques like electron microscopy, X-ray diffraction, and spectroscopy.
Crystal defects are of utmost importance in materials science as they offer avenues for tailoring material properties, enabling the design of new materials, influencing phase transformations and transport phenomena, affecting mechanical behavior, and providing valuable insights into material characterization.
Controlling and manipulating crystal defects is crucial for advancing materials science and engineering, leading to the development of innovative materials with enhanced performance and functionality.
Introduction to point defects
Point defects are one of the primary types of crystal defects that occur within materials’ crystal lattice structures. These defects involve localized deviations from the ideal arrangement of atoms or ions at specific points within the crystal lattice.
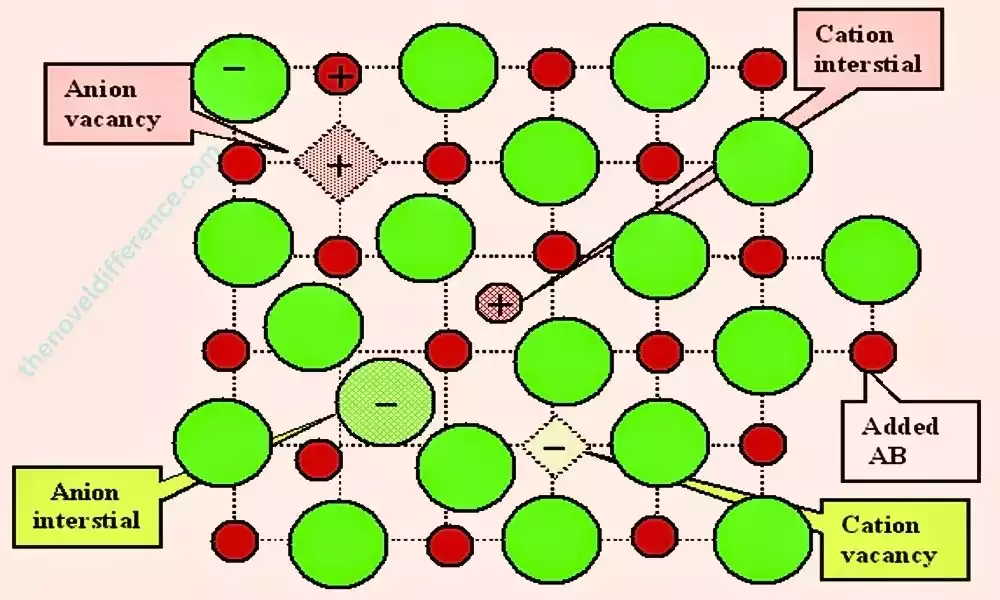
Unlike extended defects like dislocations or grain boundaries, point defects are confined to individual lattice sites and have a relatively small spatial extent.
Point defects can result from several sources, including crystal growth, temperature variations, radiation exposure, or chemical impurities. Materials test methods play a pivotal role in defining their physical, chemical, and mechanical properties. Point defects can be classified into several categories, including vacancies, interstitials, substitutional impurities, and self-interstitials.
- Vacancies: Vacancies are the simplest type of point defects and occur when a lattice site within the crystal lattice is unoccupied by an atom or ion. They result from missing atoms or ions and are characterized by a space or void at a specific lattice site. Void spaces within materials can hurt their properties such as diffusion rates, electrical conductivity, and thermal conductivity.
- Interstitials: Interstitials are point defects where atoms or ions occupy positions between the regular lattice sites. They are typically smaller than the host atoms or ions and can introduce strain or distortions to the crystal lattice. Interstitials can affect material properties such as mechanical strength, diffusion, and defect formation energies.
- Substitutional Impurities: Substitutional impurities occur when atoms or ions of a different element replace the original atoms or ions within the crystal lattice. These defects cause changes in the chemical composition of the material. Foreign atoms may alter various properties, including electrical conductivity, optical properties, and mechanical behavior, depending on their nature and concentration.
- Self-Interstitials: Self-interstitials are similar to interstitials, but they involve the displacement of atoms or ions from their regular lattice positions to occupy interstitial sites within the same crystal lattice. They can arise due to thermal activation or high-energy irradiation. Self-interstitials can affect material properties such as mechanical properties, diffusion, and defect interactions.
Point defects can significantly influence the behavior and performance of materials. They can affect atomic diffusion, electrical and thermal conductivity, mechanical strength, phase stability, and many other properties.
The concentration and arrangement of point defects within a crystal lattice can be controlled or manipulated through various techniques, including heat treatment, doping, or ion implantation, allowing for the tailoring of material properties to meet specific requirements in applications ranging from electronics and energy storage to structural materials and catalysis.
Schottky Defect
Schottky defect is a type of point defect that occurs in ionic crystals. It involves the simultaneous creation of vacancies for both cations and anions within the crystal lattice, resulting in a defect that maintains electrical neutrality. This defect is named after the German physicist Walter H. Schottky, who extensively studied point defects in crystals.
Characteristics of Schottky Defect:
- Vacancies: Schottky defects result in the creation of vacancies, which are empty lattice sites where atoms or ions are missing. These vacancies are formed in equal numbers for both cations and anions, ensuring the crystal retains its overall electrical neutrality.
- Stoichiometric Deviation: Schottky defects lead to non-stoichiometric compositions in the crystal lattice. The ratio of cations to anions deviates from the ideal stoichiometric ratio due to the absence of some atoms or ions.
- High Concentration in Ionic Compounds: Schottky defects are commonly observed in ionic compounds that have high coordination numbers, such as alkali halides (e.g., NaCl, KBr), oxides (e.g., MgO, CaO), and fluorides (e.g., AgF, PbF2). These compounds tend to have closely packed crystal structures with a large number of lattice sites.
Formation Mechanism:
Schottky defects can form through various mechanisms:
- Crystal Growth: Schottky defects can occur during the growth of an ionic crystal if there is insufficient availability of cations or anions to occupy all the lattice sites.
- Thermal Activation: At elevated temperatures, atoms or ions can overcome the energy barrier and migrate from their lattice positions, creating vacancies. The concentration of Schottky defects increases with increasing temperature.
Effects on Crystal Structure and Properties:
The presence of Schottky defects can have several effects on the crystal structure and properties:
- Density Reduction: The creation of vacancies leads to a reduction in the crystal’s density since some lattice sites remain unoccupied.
- Electrical Conductivity: Schottky defects enhance the electrical conductivity of ionic crystals. The vacancies allow for the movement of ions, increasing the availability of charge carriers and facilitating the flow of electric current.
- Ionic Conductivity: The migration of ions through the created vacancies contributes to ionic conductivity in solid electrolytes. This property is crucial for applications such as fuel cells and batteries.
- Melting Point Depression: Schottky defects lower the melting point of the crystal. The presence of vacancies disrupts the regular crystal lattice, reducing the energy required for the transition from a solid to a liquid state.
Examples and Applications:
Schottky defects have practical implications in various fields and applications, including:
- Solid-State Electronics: Schottky defects influence the electrical behavior of ionic materials used in devices like solid-state batteries, sensors, and electronic components.
- Catalysis: The presence of Schottky defects on the surface of catalysts can modify their reactivity, leading to improved catalytic activity for various chemical reactions.
- Radiation Detection: Alkali halide crystals with Schottky defects are commonly used in radiation detectors due to their ability to capture and release charge carriers upon exposure to ionizing radiation.
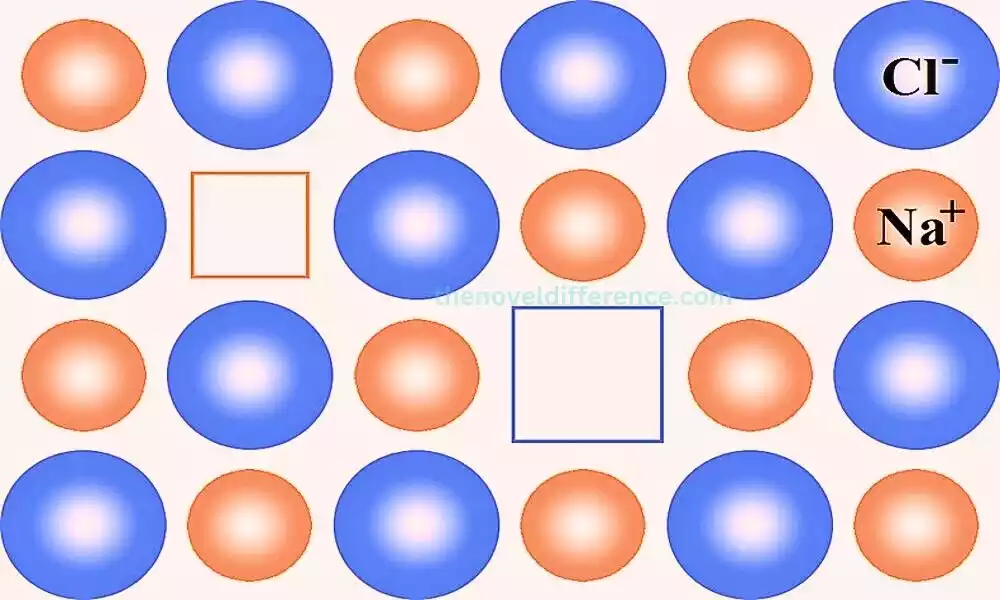
Schottky defects involve the creation of vacancies for both cations and anions in ionic crystals. These defects affect the crystal structure, electrical and ionic conductivity, and melting point. Understanding and controlling Schottky defects is crucial for tailoring material properties and optimizing their performance in various technological applications.
Frenkel Defect
Frenkel defect is a type of point defect that occurs in crystal structures, particularly in ionic crystals. It involves the displacement of an ion from its normal lattice site to an interstitial position within the crystal. The Frenkel defect is named after the Russian scientist Yakov Frenkel, who extensively studied point defects in crystals.
Characteristics of Frenkel Defect:
- Interstitial Displacement: In a Frenkel defect, an atom or ion moves from its original lattice site and occupies an interstitial position within the crystal lattice. This creates an empty lattice site at the original position and an additional atom or ion in the interstitial position.
- No Change in Charge: Frenkel defects do not introduce any net charge into the crystal since the displaced atom or ion remains within the crystal structure. Therefore, the crystal lattice maintains its overall electrical neutrality.
- Common in Compounds with Low Coordination Numbers: Frenkel defects are often observed in materials with low coordination numbers, such as metal halides (e.g., AgCl, AgBr) and metal oxides (e.g., ZnO, Cu2O). These materials have less densely packed crystal structures, allowing atoms or ions to easily occupy interstitial positions.
Formation Mechanism:
Frenkel defects can occur through various mechanisms:
- Thermal Activation: At elevated temperatures, atoms or ions in the crystal lattice can gain enough thermal energy to overcome the energy barrier and move to interstitial positions. This thermal activation allows the creation of Frenkel defects.
- Radiation: High-energy radiation, such as ion beams or X-rays, can displace atoms or ions within the crystal lattice, creating Frenkel defects. This mechanism is often observed in irradiated materials.
Effects on Crystal Structure and Properties:
The presence of Frenkel defects can have several effects on the crystal structure and properties:
- Lattice Expansion: Frenkel defects introduce additional atoms or ions into interstitial positions, leading to a slight expansion of the crystal lattice. This expansion can affect material properties such as density, lattice parameter, and thermal expansion coefficient.
- Electrical Conductivity: Frenkel defects can influence electrical conductivity in materials, especially when the displaced ions are mobile within the crystal lattice. The presence of interstitial ions provides additional charge carriers, enhancing electrical conductivity.
- Optical Properties: Frenkel defects can affect the optical properties of materials. The displacement of ions within the crystal lattice can alter the absorption, emission, and scattering of light, leading to changes in the material’s optical behavior.
- Mechanical Properties: Frenkel defects can influence the mechanical properties of materials. Introducing interstitial atoms or ions can hinder dislocation motion and affect the material’s plasticity, hardness, and strength.
Examples and Applications:
Frenkel defects have practical implications in various fields and applications, including:
- Optoelectronics: Materials with Frenkel defects are used in optoelectronic devices, such as photovoltaic cells, light-emitting diodes (LEDs), and lasers. The defects can control light absorption, emission, and transport within the materials.
- Ion Conduction: Frenkel defects contribute to ionic conductivity in solid-state electrolytes used in batteries, fuel cells, and electrochemical devices. The movement of ions within the crystal lattice, including interstitial positions, facilitates ion conduction.
- Radiation Damage: Frenkel defects are a consequence of high-energy radiation, such as nuclear radiation or particle beams. They play a role in radiation damage and the response of materials to irradiation.
Frenkel defects involve the displacement of ions from their original lattice sites to interstitial positions within the crystal lattice.
These defects affect the crystal structure, electrical conductivity, optical properties, and mechanical behavior of materials. Understanding and controlling Frenkel defects is important for tailoring material properties and optimizing their performance in various applications in optoelectronics, ion conduction, and radiation-related fields.
Comparison between Schottky and Frenkel Defects
Schottky and Frenkel defects are two types of point defects that occur in crystal structures, particularly in ionic crystals. While both defects involve deviations from the ideal crystal lattice, they differ in their formation mechanisms, characteristics, and effects on crystal properties.
Here is a comparison between Schottky and Frenkel defects:
Formation Mechanism:
- Schottky Defect: Schottky defects are formed by the creation of vacancies for both cations and anions within the crystal lattice. They can occur during crystal growth or as a result of thermal activation.
- Frenkel Defect: Frenkel defects arise from the displacement of an atom or ion from its lattice site to an interstitial position within the crystal lattice. They can occur through thermal activation or under high-energy radiation.
Characteristics:
- Schottky Defect:
- Involves the creation of vacancies for both cations and anions.
- Maintains electrical neutrality within the crystal lattice.
- Leads to a non-stoichiometric composition in the crystal lattice.
- Commonly observed in ionic compounds with high coordination numbers.
- Frenkel Defect:
- Involves the displacement of an atom or ion to an interstitial position.
- Maintains electrical neutrality within the crystal lattice.
- Does not introduce vacancies or alter the stoichiometry.
- Commonly observed in ionic compounds with low coordination numbers.
Effects on Crystal Properties:
- Schottky Defect:
- Reduces the density of the crystal due to the creation of vacancies.
- Enhances electrical conductivity by providing mobile charge carriers.
- Reduces the melting point of the crystal due to the disruption of the lattice.
- Commonly found in solid electrolytes and materials used in radiation detection.
- Frenkel Defect:
- Slightly expands the crystal lattice due to the introduction of interstitial atoms or ions.
- Can enhance electrical conductivity if the displaced ions are mobile.
- Can affect the mechanical properties and optical behavior of the material.
- Commonly found in materials used in optoelectronic devices and ion conduction applications.
Occurrence:
- Schottky Defect:
- Commonly observed in ionic compounds with high coordination numbers, such as alkali halides, oxides, and fluorides.
- Frenkel Defect:
- Commonly observed in ionic compounds with low coordination numbers, such as metal halides and metal oxides.
Schottky defects involve the creation of vacancies for both cations and anions, while Frenkel defects involve the displacement of atoms or ions to interstitial positions.
Schottky defects affect crystal density, electrical conductivity, and melting point, while Frenkel defects slightly expand the lattice and can influence electrical conductivity, mechanical properties, and optical behavior.
Understanding and controlling these defects is crucial for tailoring material properties and optimizing their performance in various applications.
Similarities Between Schottky Defect and Frenkel Defect
While Schottky and Frenkel’s defects are distinct types of point defects with different characteristics, there are some similarities between them.
These similarities include:
- Point Defect Nature: Both Schottky and Frenkel defects are classified as point defects since they occur at specific atomic or ionic positions within the crystal lattice. They are localized deviations from the ideal crystal structure.
- Electrical Neutrality: Both defects maintain the overall electrical neutrality of the crystal lattice. In Schottky defects, the creation of vacancies for both cations and anions ensures that the charges remain balanced. In Frenkel defects, the displaced atom or ion remains within the crystal lattice, preserving electrical neutrality.
- Influence on Material Properties: Both defects can influence various properties of materials. They can affect electrical conductivity, albeit through different mechanisms. Schottky defects enhance electrical conductivity by providing mobile charge carriers, while Frenkel defects may influence conductivity if the displaced ions are mobile. Additionally, both defects can affect other properties such as mechanical behavior, thermal expansion, and optical properties.
- Occurrence in Ionic Compounds: Both Schottky and Frenkel defects are commonly observed in ionic compounds. While they occur in different types of ionic materials (high coordination for Schottky and low coordination for Frenkel), their presence in ionic crystals demonstrates the importance of point defects in these materials.
- Influence on Crystal Structure: Both defects can introduce deviations and distortions to the regular crystal lattice. Schottky defects result in a non-stoichiometric composition due to the creation of vacancies. Frenkel defects slightly expand the lattice due to the introduction of interstitial atoms or ions. These structural modifications can have implications for various material properties.
Despite their similarities, it is important to note that Schottky and Frenkel’s defects have distinct characteristics, formation mechanisms, and effects on crystal properties. Understanding these differences is crucial for tailoring material properties and optimizing their performance in specific applications.
Conclusion
Schottky and Frenkel defects are two types of point defects that occur in crystal structures. Schottky defects involve the creation of vacancies for both cations and anions, while Frenkel defects involve the displacement of atoms or ions to interstitial positions. Both defects play significant roles in determining the properties and behavior of materials.
Schottky defects are characterized by the creation of vacancies, non-stoichiometric compositions, and their occurrence in materials with high coordination numbers. They can enhance electrical and ionic conductivity, reduce the density of the crystal, and lower the melting point.
Schottky defects are commonly observed in ionic compounds and have applications in solid-state electronics, catalysis, and radiation detection.