Introduction of Distillation and Chromatography
Distillation is a separation process that relies on variations in boiling points to differentiate elements in a mix. It’s extensively used for purifying liquids and for separating volatile substances.
Chromatography is a flexible lab method that can be utilized to analyze and separate mixtures based on their interactions with a mobile and stationary phase. It’s useful in many areas, ranging from chemistry to forensics, to provide precise analysis and separation of the components.
What is Distillation?
Distillation is a process of separation that is used to separate or purify elements of a mix by comparing the temperature at which they boil. It is a popular method in chemistry, industry as well and laboratory settings to purify liquids and to separate components from liquid mixtures. Distillation is based on the notion that the different components in the mixture will evaporate at different temperatures.
The way distillation is done:
- Vaporization: This mixture could include several different components that have different boiling points and temperatures, and is heated in a vessel known as a distillation flask, or boiler. As the mixture heats the component with lower boiling temperatures is likely to begin to evaporate first.
- Condensation: the vaporized elements are pushed through a cooling column which is often referred to as an evaporation column or a fractionating column. The column is made up of many packing materials, or trays which allow for several stages of vaporization and condensation. As the vapor rises it cools, and when it has reached an appropriate height within the column is greeted by a cooling system, usually one that is a condenser. The cooling system makes the vapor condense to form a liquid.
- collection: The condensed liquid is stored in a separate container known as the distillation or receiver flask. The liquid that is collected is enhanced with the component with the lowest boiling temperature and evaporated first. The process can be repeated many times, particularly in fractional distillation, to sort and separate the various elements of the mix by their boiling point.
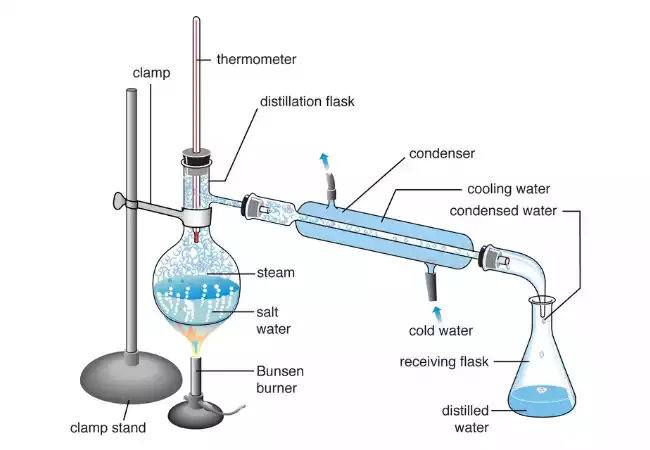
Distillation is a crucial process in many industries, which includes the production of alcoholic drinks and refineries for petroleum, chemical production, and the removal of essential oils among other things. Different kinds of distillation techniques like simple distillation or fractional distillation are used according to the specific properties of the mixture as well as the result desired.
What is Chromatography?
Chromatography is a method in the laboratory that is used to analyze and separate mixtures of substances based on their different interactions between a mobile and stationary phase. The technique is used extensively in a variety of fields, such as the fields of biochemistry, chemistry, forensic sciences, pharmaceuticals, and environmental science to assist in identifying and quantifying the constituents of a complicated mixture.
Let’s see how the chromatography functions:
- Stationary Phase: In chromatography, there’s a stationary component, which could be a solid or liquid, impermeable upon a solid base. The stationary phase may be different in its characteristics, including size or polarity. These may interact in a specific way with components of the mixture that are being separated.
- Mobile Phase: It is liquid (usually gas or liquid) that transports the sample throughout its stationary stage. The sample is placed into the mobile phase and it moves throughout the stationary taking the components with it.
- Separation Mechanism: When the cell phase passes across the stationary various components of the mixture are in contact with the stationary in different levels. These interactions could include partitioning, adsorption, or affinity, based on the kind of chromatography used.
- Separation and Detection: The components of the mixture are separated by the stationary stage at various speeds, resulting in their separation. When the components dissolve and are detected and quantified using different detection techniques including UV-visible spectroscopy, fluorescence mass spectrometry, or measures of refractive index.
The types of chromatography are:
- Thin-Layer Chromatography (TLC): In TLC the thin layer of stationary phase is deposited on the flat support. The sample is positioned as a single spot, and separation occurs as the mobile phase is moved upwards on the plate. TLC is typically used to conduct rapid qualitative tests.
- Gas Chromatography (GC): In GC it is the case that mobile phases are liquid, as is the stationary one, which is a hot boiling liquid that is encased on solid support. It is employed to separate volatile gaseous compounds.
- HDLC (HPLC): HPLC utilizes the liquid phase that is under high pressure. It can be used to study a broad range of chemicals which makes it suitable for the analysis of quantitative quantities.
- Liquid Chromatography (LC): LC uses a variety of techniques that utilize liquid mobile phases, including reverse phase, normal phase, and ion exchange which are each suitable for particular kinds of compounds.

Chromatography permits the identification and separation of specific components within a mix which makes it an essential instrument for analytical chemistry and different industrial and scientific applications.
Importance of separating mixtures in various industries
Separating mixtures is essential in many industries for a variety of reasons:
- Quality Control: The separation of mixed substances ensures that the product meets specific standards of quality. Through the removal of impurities or unneeded components, industries can create products that have consistent quality and features. This is essential in industries like pharmaceuticals and food in which purity and safety are crucial.
- Effectiveness of Resources: Processes for separation can aid in recovering valuable resources from mixed materials. For instance, in the mining and metallurgical industry, separation methods can be used to remove valuable minerals from ore, thus reducing consumption and increasing the utilization of resources.
- Waste Reduction: Separating the mixtures decreases pollution and waste production. By recycling and isolating useful components, industries can reduce the amount of polluting byproducts and harmful pollutants which contribute to environmental sustainability and sustainability.
- Production Development: For industries as diverse as materials science and pharmaceuticals precise separation methods are vital in the development and research of products. Engineers and scientists utilize separation to purify, isolate, and analyze new compounds which leads to the development of novel products.
- Energies Saved: Separation processes often require energy-intensive steps like distillation or filtration. Effective separation methods can result in savings in energy and costs. For instance, the petroleum industry relies on distillation techniques to divide crude oil into various products, which helps to maximize energy usage.
- Security: In the chemical industry, the separation of the compounds is vital to ensure security. By purifying or isolating hazardous chemical substances, the danger of chemical reactions and accidents is decreased. This is especially important when handling explosive or toxic substances.
- pharmaceuticals: Pharma industry uses separation methods to isolate the drug molecules, assuring their safety and efficacy. Separation also plays an essential part in the production of vaccines and allows the separation of proteins and antigens.
- Food and beverages: Separation processes are employed to eliminate contaminants, isolate ingredients as well and preserve the taste and quality of drinks and food. These processes aid in the safety of food and ensure quality.
- Environmental Remediation: In the field of environmental remediation Separation techniques are used to eliminate pollutants from water, air, and soil. This aids in the cleanup of sites that have been contaminated and to protect the natural environment.
- Biotechnology: Separation in Biotechnology is essential, as it helps purify biomolecules, such as proteins, DNA, and enzymes. The purified components are crucial in a myriad of biotechnological uses, which include genomic engineering as well as diagnostics.
- Chemical production: Chemical production employs separation to make as well as isolate chemical substances, thus ensuring the required purity and quality. Separation is the most important element in the manufacturing of a broad range of chemicals, such as polymers, solvents, and special chemicals.
- Materials Engineering: In the field of engineering and materials, separation procedures are employed to remove and refine the raw materials, which results in better materials with improved properties. This is crucial in the development of high-tech materials, composites, as well as alloys.
The importance of separating the various types of mixtures has many facets, including the control of quality and sustainability, the efficiency of resource security, the development of products, as well as energy savings. The ability to isolate and separate components from mixtures is a crucial element of industrial processes across many industries.
Key comparison chart
Here’s a key comparison chart highlighting the main differences between distillation and chromatography:
| Aspect | Distillation | Chromatography |
|---|---|---|
| Principle | Separation is based on differences in boiling points. | Separation is based on differential interactions with a mobile phase and a stationary phase. |
| Phases | Two phases: liquid (or solid) stationary phase and vapor (or gas) mobile phase. | Two phases: stationary phase (solid or liquid) and mobile phase (liquid or gas). |
| Process | Vaporization and condensation. | Elution through the stationary phase. |
| Types | Simple distillation, fractional distillation, etc. | Thin-layer chromatography (TLC), gas chromatography (GC), high-performance liquid chromatography (HPLC), etc. |
| Applications | Purification of liquids, and separation of volatile components. | Analyzing complex mixtures, separating and identifying compounds. |
| Selectivity | Based on boiling points. | Based on interactions (e.g., affinity, size, polarity). |
| Sample State | Typically works with liquids or liquids that can be vaporized. | Works with a wide range of sample states (liquids, gases, solids). |
| Efficiency | Generally less efficient for complex mixtures. | More efficient for complex mixtures. |
| Cost and Complexity | May be simpler and cost-effective for certain applications. | Often more complex and costly, but versatile. |
| Purity | Purity is influenced by boiling points and efficiency. | Purity can be controlled and improved by adjusting conditions. |
| Time Required | May take longer for some separations. | Faster for many separations. |
| Industries | Commonly used in the petrochemical industry. | Widely used in analytical chemistry, pharmaceuticals, food, and more. |
| Energy Consumption | Uses significant energy for heating and vaporization. | Typically consumes less energy. |
| Safety | Can involve high temperatures and pressurized systems. | Generally safer, especially in laboratory settings. |
| Environmental Impact | May generate more waste and emissions. | Tends to be more environmentally friendly. |
This chart provides a concise comparison of the key differences between distillation and chromatography in terms of their principles, applications, efficiency, and other important aspects. The choice between these techniques depends on the specific needs of a given separation process and the nature of the mixture being dealt with.
Real-World Examples
Here are some real-world examples of chromatography and distillation applications:
Distillation:
- petroleum Refining: Crude oil is refined in refineries to break it down into various components, including diesel, gasoline, and kerosene, based on the boiling point of their respective components.
- Alcohol Production: Distillation plays a role in making alcohol-based drinks such as vodka and whiskey to isolate alcohol from fermented mixtures.
- Desalination Distillation is utilized to desalinate seawater, in which the water evaporates leaving behind impurities and salts.
- Pharmaceuticals: Distillation helps separate and purify different elements in the manufacturing of pharmaceuticals, for example, the isolation of certain active components.
- Chemical Synthesis: Within the chemical industry distillation is employed to remove and remove chemical compounds for a variety of uses, such as the manufacture of special chemicals.
Chromatography:
- Forensic Analysis: Gas chromatography can be utilized to identify and separate volatile substances in forensic investigation, like the detection of explosive residues or drugs.
- Environmental Monitoring: Liquid Chromatography is used to analyze soil and water samples for pollutants such as pesticides and heavy metals.
- Food and Beverage Industry: Chromatography is used to identify the ingredients in flavorings, food additives, and other contaminants, assuring the safety of your food and its quality.
- The process of drug testing: Chromatography is used in drug testing laboratories to determine and quantify the presence of the presence of drugs or their metabolites in biological samples such as blood or urine.
- Biotechnology: High-performance liquid chromatography (HPLC) is employed in biotech to purify as well as analysis of protein as well as nucleic acids. It is essential to the creation of biopharmaceuticals.
- pharmaceutical quality control: Chromatography can be used to test the quality of pharmaceuticals by verifying the purity and presence of active components.
- Oil and Gas Industry: Gas chromatography is used to measure and analyze the impurities and hydrocarbons in petroleum products and natural gas.
These real-world examples illustrate the numerous applications of chromatography and distillation across various industries and highlight their importance in ensuring the quality of products and safety as well as conformity to the industry’s standards.
Advantages of Each
These are the benefits of both chromatography and distillation:
Advantages of Distillation:
- Purity: Distillation is extremely efficient at separating substances with distinct boiling points, which results in a high level of purity within the separated components.
- Flexibility: This is utilized to purify and separate an array of liquids, including ones that contain chemical compounds with volatile properties.
- Simple: Simple distillation particularly it is a simple procedure that doesn’t require a lot of equipment.
- Scalability: Distillation is easily increased in size for industrial purposes which makes it suitable for production on a large scale.
- Resources Recovery: The HTML0 format is useful to recycle and recover solvents and useful chemicals from the waste stream.
- widely used: Distillation has a long-standing history of being used across a range of industries, and information and experience are abundantly available.
Advantages of Chromatography:
- Selectivity: Chromatography can separate complex mixtures by the highest degree of selectiveness dependent on various variables such as size, polarity, and affinity.
- High-Resolution: Chromatography offers high-resolution separation. This allows for the analysis and identification of the individual elements in complicated mixtures.
- Wide Application: Chromatography techniques like HPLC and GC can be applied to a vast spectrum of different types of samples such as liquids, gases, and solids.
- Analytical Capability: Chromatography is an effective analytical tool that is often employed for the quantification and characterization of compounds.
- Minimum Sample Preparation: This typically requires less sample preparation than distillation, which is labor-intensive and time-consuming.
- Environmentally friendly: Chromatography generally produces less waste and also has less environmental impact than other distillation methods.
- Safety: It is generally regarded as more secure, particularly in lab settings since it doesn’t require high temperatures.
- Automation: Numerous systems for chromatography can be completely automated, which eliminates the requirement for continuous operator involvement.
- Peak collection: Chromatography may identify peaks, fractions, or peaks of interest, which makes it a good method for isolating particular compounds.
The decision to choose between distillation or chromatography is based on the particular needs of the separation process and the specific characteristics of the mix. Both methods have advantages and drawbacks, so choosing the right method is vital to get the desired outcomes efficiently and efficiently.
Limitations of Each
The distillation process and the chromatography have limitations. Understanding these limitations is vital to choosing the best method to accomplish a separation job.
These are restrictions of each of the following:
Limitations of Distillation:
- Complex Mixtures: Distillation can be less efficient when it comes to complicated mixtures that contain components with similar boiling points because it might not be able to provide enough separation.
- Destruction of Heat-Sensitive Compounds: Certain heat-sensitive compounds may disintegrate during distillation due to the extreme temperatures that are involved.
- Energy intensive: Distillation can be intensive in terms of energy, particularly for high-temperature separations, and could not be economically feasible in certain circumstances.
- Scale-Up Challenges: While distillation is an easily adaptable process, it can become more complicated and expensive at a higher scale because of increased power requirements and costs for equipment.
- A high initial investment: The process of setting up distillation equipment, specifically for processes that require specialized expertise, such as fractional distillation, requires a significant initial investment.
- Trouble in Separating Azeotropes: Azeotropes, mixtures of constantly boiling temperatures, are difficult to separate using simple distillation.
- Limited Application to Solid Mixtures: Distillation is most suited for liquids and is not efficient for separating solid mixtures.
Limitations of Chromatography:
- Small Size of Sample: Chromatography often requires very small samples This could be a drawback when only small quantities of a sample can be obtained.
- Complexity and Costs: High-performance chromatography systems are often complex and expensive which requires skilled operators and substantial maintenance.
- Resolution Problems: Achieving high resolution in chromatography may be difficult and may require sophisticated equipment and optimization.
- The possibility of contamination: In certain situations, chromatographic columns could be contaminated or soiled which can affect separation efficiency.
- Modification: Chromatography results can be affected by factors such as column temperature flow rate, column temperature, and the level of operator proficiency which is why it is important to keep the same results.
- A limited application for high-boiling compounds: Certain chromatography techniques are less efficient for high-boiling compounds due to the potential for overloading the column.
- Longer Analysis Time: Chromatographic analyses may be more time-consuming than distillation, which could be a problem in situations that require time.
- A lifetime of Column: The columns used in chromatography have a short lifespan require replacement regularly and can result in operating costs.
- Safety Concerns: While generally safer than distillation using certain chemicals and solvents used in chromatography may still raise security concerns if treated properly.
The decision between distillation and chromatography is based on the particular requirements of the separation process and the specific characteristics of the mix, as well as different practical considerations like time, cost, as well as available resources. Knowing how each method is vital to making an informed choice.
Conclusion
The distillation process and the chromatography serve as essential separation methods used in many sciences and industries. Distillation is a great method of separating the components based on the difference in boiling points. It provides the highest purity, but it has limitations in handling complicated mixtures and the energy consumed.
The chromatography process offers superior quality, selectivity, and flexibility, which makes it ideal for a broad spectrum of applications. However, it could require more complicated equipment and is more suitable for smaller samples. Selecting the best method is dependent on the particular requirements of the separation work and the specifics of the mix in question.