A brief overview of malic acid and citric acid
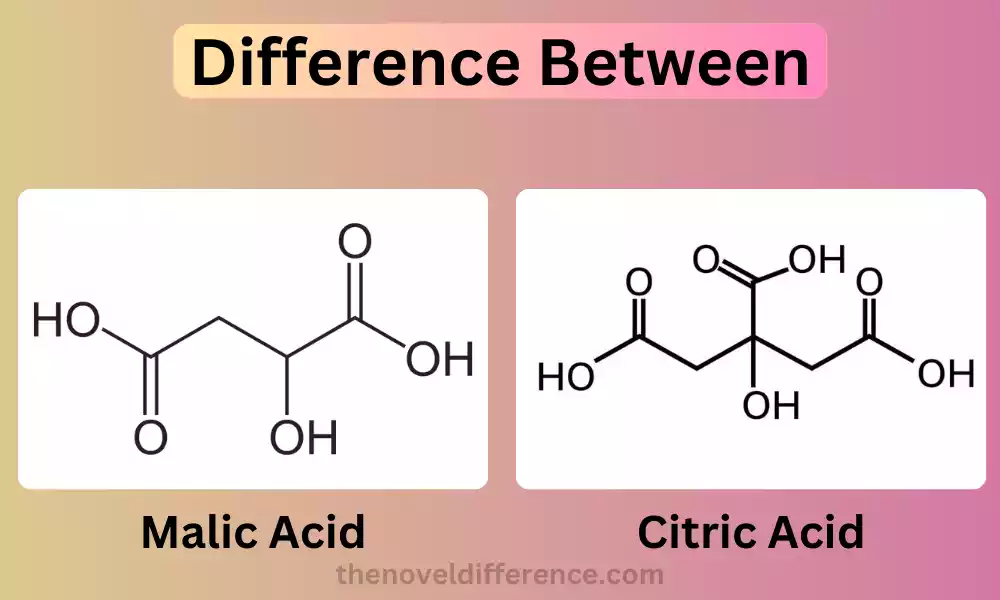
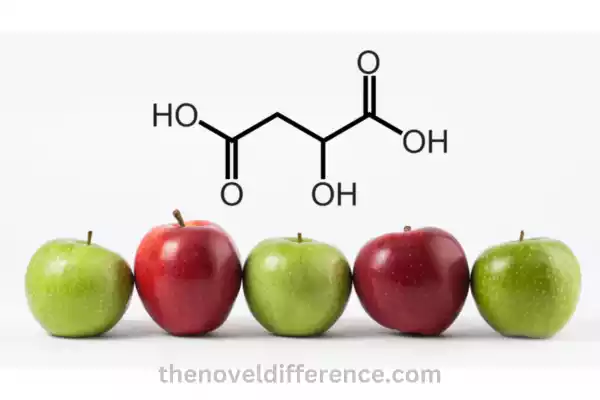
Malic Acid: Malic acid, an Organic dicarboxylic acid compound found naturally, belongs to an acid group known as dicarboxylic acids. It is found abundantly in various fruits, particularly in apples, which is why it is named “malic” acid.
It has a chemical formula of C4H6O5 and is known for its sour taste. Malic acid has an important place in food and beverage products as an acidulant and flavor enhancer, adding its tart and tangy flavors.
It also helps in pH adjustment and acts as a natural preservative. Besides its culinary uses, malic acid has potential health benefits and is used in medicine as an energy production aid and a possible antioxidant.
Citric Acid: Citric acid, which is naturally found in citrus fruits like citrus fruits Lemons, and grapefruits is well-known for its crucial roles in their structure as well as preservation. Its chemical formula is C6H8O7.
Citric acid has a refreshing and tangy taste, which gives citrus fruits their characteristic sourness. In the food and beverage industry, citric acid serves as an Acidulant and souring agent, contributing to the tartness of various products. It also acts as a flavor enhancer and preservative.
Beyond its culinary applications, citric acid has health benefits and is used in medicine to aid digestion, boost metabolism, and potentially exhibit antioxidant and antimicrobial properties.
Acid and malic acid are two chemicals widely utilized across various industries, from food and beverages, pharmaceuticals, cleaning agents, and cosmetic products to cleaning supplies and personal care items.
While they share similarities in their acidic properties and applications, they also have distinct characteristics and uses that set them apart from each other.
Importance of acids in various industries and applications
Acids play an indispensable part in numerous industries and applications due to their unique chemical properties.
Some of the key industries where acids are important include:
1. Food and Beverage Industry:
• Acids such as citric acid, malic acid, and phosphoric acid are used as acidulants to provide a sour taste and enhance flavors in food and beverages.
• Acids act as pH regulators, preserving and stabilizing food products.
• They also serve as natural preservatives, preventing the growth of bacteria and extending the shelf life of food and beverages.
2. Pharmaceutical Industry:
• Acids are used in the production of pharmaceutical drugs as catalysts or reagents.
• They are employed in the formulation of tablets, capsules, and liquid medications to adjust pH, enhance drug stability, and improve solubility.
• Some acids, such as salicylic acid, are used in the production of topical medications and skincare products.
3. Chemical Industry:
• Acids are essential in the synthesis of various chemical compounds, including dyes, pigments, plastics, and fertilizers.
• Sulfuric, hydrochloric, and nitric acids are widely employed as chemical reaction catalysts and purification solutions in industrial manufacturing processes.
4. Cleaning and Household Products:
• Acids, such as acetic acid (vinegar) and citric acid, are commonly found in cleaning agents, disinfectants, and descaling solutions.
• They help remove stains, mineral deposits, and rust from various surfaces and equipment.
5. Metal Processing and Mining:
• Acids, such as sulfuric acid, are used in metal extraction processes, such as leaching and refining.
• They are employed in removing impurities, purifying metals, and in the production of fertilizers.
6. Water Treatment:
• Acids, like hydrochloric acid and sulfuric acid, are utilized in water treatment plants to adjust pH levels, neutralize alkaline substances, and control microbial growth.
7. Energy and Batteries:
• Acids, including sulfuric acid and phosphoric acid, are used in the production of batteries, such as lead-acid batteries commonly found in automobiles.
The importance of acids in these industries lies in their ability to modify chemical reactions, adjust pH levels, enhance product characteristics, and ensure product safety and stability. Their versatile properties make them indispensable in numerous applications, contributing to advancements and innovation in various fields.
Malic Acid
Malic acid is a naturally occurring organic compound with the chemical formula C4H6O5. It is classified as a dicarboxylic acid due to its two carboxyl (-COOH) groups.
Malic acid is found abundantly in fruits, with apples being a particularly rich source, which is why it is named “malic” acid. Vitamin B5 can also be found in other sources like grapes, cherries, and citrus fruit as well as certain vegetables.
Here are some key features and applications of malic acid:
1. Flavor Profile and Sensory Characteristics:
• Malic acid has a sour taste and contributes to the tartness and acidity of foods and beverages.
• It provides a pleasant, crisp, and refreshing sensation on the palate.
• The sourness of malic acid is often associated with green apples.
2. Role in the Food and Beverage Industry:
• Malic acid is widely used as an acidulant and flavor enhancer in the food and beverage industry.
• It adds a tangy flavor to various products such as fruit-flavored candies, beverages, and desserts.
• Malic acid is employed to balance sweetness and provide a desirable tartness in formulations.
3. pH Adjustment and Preservation:
• Malic acid helps regulate and lower pH levels in food and beverage products.
• It acts as a natural preservative, inhibiting the growth of microorganisms and extending the shelf life of products.
4. Health Benefits and Uses in Medicine:
• Malic acid is involved in the Krebs cycle (also known as the citric acid cycle) in the human body, which plays a vital role in energy production.
• It is sometimes used as a supplement to help alleviate symptoms of fibromyalgia and chronic fatigue syndrome.
• Malic acid is believed to possess potential antioxidant properties, although further research is needed to fully understand its effects.
5. Industrial Applications:
• Malic acid finds applications in the cosmetic and personal care industry, where it is used in formulations such as skin and hair care products.
• It is utilized in cleaning agents and detergents due to its chelating properties, which help remove mineral deposits and improve cleaning efficiency.
Malic acid is an integral component of many food and beverage products that enhances flavor, acidity, and preservation. Its versatile applications extend to the pharmaceutical, cosmetic, and cleaning industries, making it a valuable ingredient in numerous formulations.
Definition and chemical properties
Definition: Malic acid is an organic compound classified as a dicarboxylic acid. Apples are known to contain high concentrations of this naturally-occurring sugary compound that’s an abundant source of fructose. Chemically, malic acid is represented by the chemical formula C4H6O5.
Chemical Properties:
1. Molecular Formula: The molecular formula of malic acid is C4H6O5, indicating the presence of four carbon atoms, six hydrogen atoms, and five oxygen atoms.
2. Structure: Malic acid has a specific molecular structure characterized by two carboxyl (-COOH) functional groups. Given its two carboxyl groups, citric acid can be considered a dicarboxylic acid.
3. Acidic Nature: Malic acid is an organic acid, meaning it has acidic properties. It is considered a weak acid with a pKa value (a measure of acid strength) of around 3.4. This acidity contributes to the sour taste of malic acid-containing foods and beverages.
4. Solubility: Malic acid is highly soluble in water. It readily dissolves in aqueous solutions, forming a clear and colorless solution.
5. Chirality: Malic acid exhibits chirality, meaning it exists in two mirror-image forms known as enantiomers: L-malic acid and D-malic acid. These enantiomers have the same chemical formula but differ in their spatial arrangement of atoms. L-malic acid is the naturally occurring form found in fruits.
6. Stability: Malic acid is relatively stable under normal conditions. However, it may undergo decomposition or degradation when exposed to high temperatures or strong acids.
7. Reactivity: Malic acid can participate in various chemical reactions. For instance, it can undergo esterification reactions to form malate esters. It can also undergo oxidation reactions under specific conditions.
Malic acid’s chemical properties are key in its various applications across industries, from food and beverages, pharmaceuticals and cosmetics, through cleaning products to home maintenance. These properties determine its behavior, reactivity, and interactions in various formulations and processes.
Citric Acid
Definition: Citric acid is an organic compound with the chemical formula C6H8O7. Citrus fruits naturally contain citric acid in their fruit composition – specifically lemons, oranges and grapefruits. This weak acid also can be found in wine vinegar. Citric acid has many industrial applications due to its acidic and chelating properties.
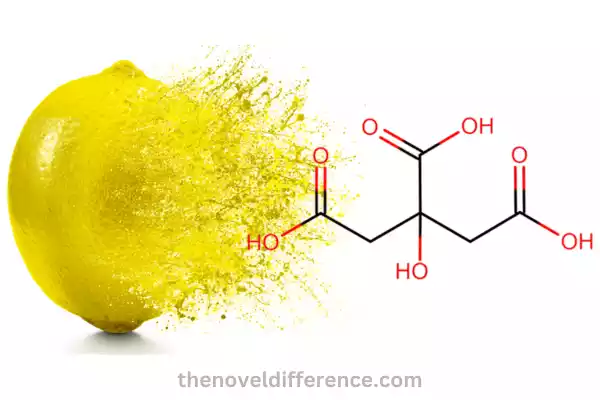
Chemical Properties:
1. Molecular Formula: The molecular formula of citric acid is C6H8O7, indicating the presence of six carbon atoms, eight hydrogen atoms, and seven oxygen atoms.
2. Structure: Citric acid has a specific molecular structure consisting of three carboxyls (-COOH) groups, making it a triprotic acid. The presence of these carboxyl groups contributes to its acidity.
3. Acidic Nature: Citric acid is a weak acid with a pKa value of approximately 3.1. It donates hydrogen ions (H+) when dissolved in water, contributing to its sour taste and acidity.
4. Solubility: Citric acid is highly soluble in water. It readily dissolves, forming a clear and colorless solution.
5. Chelating Properties: Citric acid exhibits chelating properties, meaning it has the ability to bind to metal ions. This property is utilized in various applications, such as cleaning agents and descaling solutions.
6. Crystalline Form: Citric acid is commonly found in the form of colorless crystals or a white crystalline powder. These crystalline forms are stable and easy to handle.
7. Stability: Citric acid is relatively stable under normal conditions. Decomposition at high temperatures results in the release of carbon dioxide, water and other by-products.
8. Reactivity: Citric acid can participate in various chemical reactions. It can undergo esterification, oxidation, and decarboxylation reactions under specific conditions.
Citric acid’s chemical properties make it a versatile compound with numerous applications. It is used extensively to enhance flavor, acidulant or preservative within the food and drink industry.
Due to its acidity its chelating capability and solubility, it can also be found its application in cosmetics, pharmaceuticals as well as cleaning products, as well as water treatment equipment. Understanding its chemical properties is crucial for utilizing citric acid effectively in different processes and formulations.
Definition and chemical properties
Definition: Citric acid is an organic acid with the chemical formula C6H8O7. Citrus fruits contain natural citrate compounds like lemons, oranges and grapefruits which contain this compound. Citric acid has numerous applications across industries due to its acidic, flavor-enhancing, and chelating properties.
Chemical Properties:
1. Molecular Formula: The molecular formula of citric acid is C6H8O7, indicating the presence of six carbon atoms, eight hydrogen atoms, and seven oxygen atoms.
2. Structure: Citric acid has a specific molecular structure characterized by three carboxyl (-COOH) functional groups. These groups contribute to its acidity and ability to donate hydrogen ions (H+) when dissolved in water.
3. Acidity: Citric acid is a weak acid with a pKa value of approximately 3.1. It readily donates hydrogen ions, resulting in its acidic properties.
4. Solubility: Citric acid is highly soluble in water. It dissolves easily, forming a clear and colorless solution.
5. Chelating Properties: One notable property of citric acid is its chelating ability. It can bind with metal ions, such as calcium and iron, forming soluble complexes. This property is utilized in various applications, including cleaning agents and descaling solutions.
6. Crystalline Form: Citric acid is commonly available as colorless crystals or a white crystalline powder. These crystalline forms are stable and easily handled.
7. Stability: Citric acid is stable under normal conditions. At high temperatures, however, carbon can undergo thermal decomposition into carbon dioxide, water, and various by-products.
8. Reactivity: Citric acid can participate in various chemical reactions. It can undergo esterification reactions to form citrate esters, and it can also undergo oxidation and decarboxylation reactions under specific conditions.
The chemical properties of citric acid contribute to its wide range of applications in various industries. Citric acid can be utilized as acidulants, flavor enhancers and preservatives in food and beverage manufacturing industries.
Its chelating properties find application in cleaning agents, descaling solutions, and as a food additive. Understanding its chemical properties is crucial for utilizing citric acid effectively in different processes and formulations.
Comparison between Malic Acid and Citric Acid
When comparing malic acid and citric acid, there are several key differences to consider:
1. Chemical Structure: Malic acid has the molecular formula C4H6O5, while citric acid’s formula is C6H8O7. Citric acid has a larger and more complex chemical structure with three carboxyl (-COOH) groups, while malic acid only possesses two. This structural difference contributes to variations in their properties and behavior.
2. Natural Sources: Malic acid is abundantly found in apples and other fruits, while citric acid is naturally occurring in citrus fruits such as lemons, oranges, and grapefruits. Both acids can be derived from natural sources, but their occurrence in different fruits gives them distinct flavor profiles.
3. Taste and Flavor: Malic acid is known for its sour taste and is often associated with the tartness of green apples. It provides a sharp and pronounced acidity. Citric acid also offers a tart taste, yet offers more balanced and refreshing acidity in citrus fruit products such as citrus juice. The taste difference contributes to variations in flavor profiles when used in food and beverages.
4. Acidity Strength: Citric acid is generally considered slightly stronger in acidity compared to malic acid. The pKa value of citric acid is around 3.1, while malic acid has a pKa value of approximately 3.4. This difference in acidity strength can influence their applications in terms of pH adjustment and tartness perception.
5. Applications: Both malic acid and citric acid find extensive use in the food and beverage industry. However, citric acid is more commonly used as a food additive, acidulant, and flavor enhancer in a wide range of products. It is also widely used in cleaning agents and descaling solutions due to its chelating properties. Malic acid, on the other hand, is widely utilized due to its tart flavor and acidic characteristics in various applications such as candies, beverages and products with fruit-flavored profiles.
6. Health Benefits: Both malic acid and citric acid have potential health benefits, although the specific effects may vary. Malic acid may provide an effective nutritional supplement for treating conditions like Fibromyalgia or Chronic Fatigue Syndrome. Citric acid has long been celebrated for its powerful antimicrobial and antioxidant effects. More research must be completed to better comprehend and validate these potential advantages.
While malic acid and citric acid share similarities as organic acids, their distinct chemical structures, taste profiles, and applications set them apart. The choice between malic acid and citric acid often depends on the desired flavor, acidity strength, and specific application requirements in various industries.
What are the similarities between Malic Acid and Citric Acid?
Malic acid and citric acid, despite their differences, also share some similarities:
1. Organic Acids: Both malic acid and citric acid are organic acids classified as dicarboxylic acids. They contain carboxyl (-COOH) functional groups, which contribute to their acidic properties.
2. Naturally Occurring: Both acids are found naturally in various fruits. Malic acid can be found abundantly in apples while citric acid can be found abundantly in citrus fruits such as lemons, oranges, and grapefruits.
3. Acidic Taste: Both malic acid and citric acid have a sour taste, which contributes to the tanginess and acidity in foods and beverages.
4. Flavor Enhancers: Both acids are widely used as acidulants and flavor enhancers in the food and beverage industry. They provide a tart and tangy flavor, enhancing the taste of products.
5. pH Adjustment: Malic acid and citric acid are used to adjust pH levels in food and beverage formulations. They can lower the pH, providing a desired level of acidity and helping to balance flavors.
6. Preservation: Both acids act as natural preservatives, inhibiting the growth of microorganisms and extending the shelf life of food and beverages.
7. Solubility: Malic acid and citric acid are highly soluble in water, allowing for easy incorporation into various formulations.
8. Industrial Applications: Both acids find applications beyond the food and beverage industry. They are used in cleaning agents and detergents due to their chelating properties, which help remove mineral deposits. They are also employed in the cosmetic and personal care industry for their beneficial properties.
While malic acid and citric acid have distinct characteristics, their shared properties make them versatile compounds used in various applications. Their acidic nature, flavor-enhancing abilities, and natural occurrence contribute to their importance in industries such as food, beverage, cosmetics, and cleaning products.
Malic Acid vs Citric Acid in Tabular Form
Sure! Here’s a comparison between malic acid and citric acid in tabular form:
| aspect | Malic Acid | Citric Acid |
| Chemical Formula | C4H6O5 | C6H8O7 |
| Natural Sources | Apples, grapes, cherries | Citrus fruits (lemons, oranges, grapefruits) |
| Taste | Sour, sharp | Sour, tangy, refreshing |
| Acidity Strength | Weaker (pKa ~ 3.4) | Stronger (pKa ~ 3.1) |
| Flavor Profile | Tart, green apple-like | Tangy, citrus-like |
| Applications | – Acidulant and flavor enhancer in foods and beverages |
- pH adjustment and preservation
- Energy production aid | – Acidulant and flavor enhancer in foods and beverages
- pH adjustment and preservation
- Chelating agent in cleaning products | | Industrial Uses | Cosmetic and personal care products, cleaning agents | Cleaning agents, descaling solutions | | Health Benefits | Potential energy production aid, possible antioxidant properties | Potential antioxidant and antimicrobial properties | | Molecular Structure | Dicarboxylic acid with two carboxyl groups (-COOH) | Triprotic acid with three carboxyl groups (-COOH) | | Solubility | Highly soluble in water | Highly soluble in water | | Chirality | No | No |
This table compares malic and citric acids by outlining some key differences, including chemical formula, natural sources, taste, acidity strength and flavor profile as well as applications, industrial uses, health benefits as well as molecular structure solubility chirality.
Conclusion
Malic and citric acids are two organic acids that play an integral part in various industries and applications, contributing significantly to various industries’ operations and processes.
Malic acid can be found abundantly in apples and other fruits and provides a unique sour and tart taste, making it popular as an acidulant, flavor enhancer, and pH adjuster in food and beverage production. Malic acid also has potential health benefits and applications in medicine, cosmetics, and cleaning products.
Citric acid, found naturally in citrus fruit, imparts an irresistibly tart and refreshing taste that’s widely utilized as an acidulant, flavor enhancer, and preservative in food and beverage industry products and as cleaning solutions and descaling agents. With such strong properties that make its application extremely beneficial.