Various terms and equations are used to describe and quantify chemical reactions. Two such terms are Ksp and Keq, which are often used interchangeably but have distinct meanings and applications. Understanding the differences between Ksp and Keq is essential for grasping the fundamentals of chemical equilibrium and solubility. This article aims to delve into the disparities between Ksp and Keq, shedding light on their definitions, applications, and significance in the world of chemistry.
Importance of equilibrium constants in chemistry
Equilibrium constants play a crucial role in chemistry as they provide valuable information about the state of a chemical reaction at equilibrium.
Here are some important points highlighting the significance of equilibrium constants:
1. Predicting the direction of a reaction: Equilibrium constants, such as Keq, indicate the relative concentrations of reactants and products at equilibrium. By comparing the value of Keq to 1, chemists can determine whether the reaction predominantly favors the reactants (Keq < 1) or the products (Keq > 1). This information helps in predicting the direction in which a reaction will proceed under specific conditions.
2. Assessing the extent of a reaction: The magnitude of the equilibrium constant reflects the degree to which a reaction reaches equilibrium. A large Keq value indicates that the reaction proceeds nearly to completion, while a small Keq value suggests that the reaction is relatively incomplete. This knowledge is useful in evaluating the efficiency of reactions and understanding the equilibrium position.
3. Determining optimal reaction conditions: Equilibrium constants are influenced by factors such as temperature and pressure. By studying the effect of these variables on Keq, chemists can determine the conditions that maximize the production of desired products or favor a specific reaction pathway. This information is critical for designing and optimizing chemical processes.
4. Quantitative analysis: Equilibrium constants allow for quantitative analysis of chemical systems. They provide a means to relate the concentrations or pressures of reactants and products at equilibrium, enabling the calculation of unknown quantities in equilibrium systems. Equilibrium constants enable precise calculations of equilibrium concentrations, helping chemists understand the composition of a system.
5. Comparing different reactions: Equilibrium constants provide a basis for comparing the relative positions of different chemical reactions. By comparing the values of Keq for various reactions, chemists can determine which reactions are more likely to proceed to completion, are more balanced, or are favored under specific conditions. This knowledge aids in understanding the relative strengths of different chemical reactions.
6. Guiding equilibrium manipulation: Equilibrium constants guide the manipulation of chemical equilibria. By understanding the factors that affect Keq, such as temperature or the addition of catalysts, chemists can modify reaction conditions to favor the desired product or optimize reaction yields. Equilibrium constants provide a theoretical framework for making informed decisions in chemical synthesis and industrial processes.
Equilibrium constants are essential tools in chemistry as they provide a quantitative understanding of chemical equilibria. They assist in predicting reaction directions, assessing reaction extents, determining optimal conditions, enabling quantitative analysis, facilitating comparisons between reactions, and guiding equilibrium manipulation. Equilibrium constants enhance our understanding of chemical systems and drive advancements in various fields, including pharmaceuticals, materials science, and environmental chemistry.
Definition of Ksp and Keq
Ksp (Solubility Product Constant): The dissolvability item consistently signified as Ksp, is a harmony consistently particularly utilized to portray the dissolvability of a sparingly solvent compound in a dissolvable. It represents the equilibrium between the dissolved ions and the undissolved solid compound in a saturated solution at a specific temperature. Ksp is calculated by multiplying the concentrations of the ions raised to their stoichiometric coefficients in the balanced chemical equation for the dissolution of the compound.
Keq (Equilibrium Constant): The equilibrium constant, denoted as Keq, is a measure of the extent to which a chemical reaction reaches equilibrium. It speaks to the proportion of the item concentrations (raised to their stoichiometric coefficients) to the reactant concentrations (too raised to their stoichiometric coefficients) at balance. Keq is calculated based on the balanced chemical equation for the reaction and is independent of the initial concentrations or pressures of the reactants and products. It provides information about the relative concentrations of reactants and products at equilibrium and can indicate the direction in which the reaction predominantly proceeds.
It’s important to note that Ksp is specific to solubility equilibrium, while Keq is used to describe the equilibrium state of any chemical reaction. Ksp focuses on the dissolution of a compound in a solvent, while Keq encompasses a wider range of reactions, including gas-phase reactions, homogeneous and heterogeneous reactions, and acid-base reactions, among others.
What is Ksp?
Ksp stands for Solubility Product Constant. It could be a particular sort of balance steady that’s utilized to depict the dissolvability of a sparingly dissolvable compound in water or any other dissolvable.
When a strong compound is put in a dissolvable, it may separate into its constituent particles, shaping a soaked arrangement. The solubility product constant, Ksp, quantitatively represents the equilibrium between the dissolved ions and the undissolved solid at saturation.
The Ksp value is calculated by multiplying the concentrations of the dissociated ions raised to the power of their stoichiometric coefficients. The expression for the solubility product constant is derived from the balanced chemical equation for the dissolution reaction.
Ksp is temperature-dependent, meaning that its value changes with temperature. Higher temperatures generally result in increased solubility and higher Ksp values for most compounds.
The magnitude of the Ksp value provides information about the maximum amount of solute that can dissolve in the solvent. The next Ksp esteem shows a more solvent compound, whereas a lower Ksp esteem proposes a less dissolvable compound.

By comparing the actual ion concentrations in a saturated solution with the calculated Ksp value, it is possible to determine whether a particular compound will precipitate or remain dissolved in a given solvent.
What is Keq?
Keq stands for Equilibrium Constant. It is a general term for an equilibrium constant that represents the ratio of the concentrations (or pressures) of products to reactants at a specific temperature.
When a chemical reaction reaches equilibrium, the forward and reverse reactions occur at the same rate, and the concentrations (or pressures) of reactants and products remain constant. The equilibrium constant, Keq, quantitatively describes the state of the reaction at equilibrium.
The Keq value is calculated by writing the balanced chemical equation for the reaction and using the concentrations (or pressures) of the species at equilibrium. Each species’ concentration (or pressure) is raised to the power of its stoichiometric coefficient in the balanced equation, and the resulting values are multiplied together to obtain Keq.
The magnitude of the Keq value provides information about the relative amounts of products and reactants at equilibrium. A Keq esteem more noteworthy than 1 demonstrates that the response favors the arrangement of items, whereas a Keq esteem less than 1 proposes that the response favors the arrangement of reactants. If the Keq value is close to 1, it indicates that the reaction is balanced, with roughly equal concentrations (or pressures) of products and reactants at equilibrium.
Keq is independent of the initial concentrations (or pressures) of the reactants and products. It as it were depends on the temperature at which the response is carried out. Different reactions have different Keq values, reflecting their equilibrium compositions.
Keq can be utilized to foresee the course in which a response will continue under certain conditions. If the reaction is not at equilibrium, it will naturally proceed in the direction that will establish equilibrium and adjust the concentrations (or pressures) of the species involved. By comparing the initial concentrations (or pressures) to the Keq value, one can determine which way the reaction will shift to reach equilibrium.
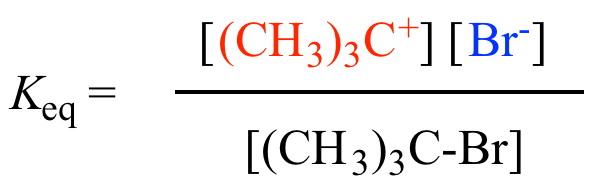
The Keq value provides crucial information about the equilibrium composition of a reaction, allowing chemists to understand the relative amounts of reactants and products and predict the direction of the reaction under specific conditions.
Differences between Ksp and Keq
There are several key differences between Ksp and Keq:
1. Definition and application:
• Ksp: It describes the equilibrium between a sparingly soluble compound and its dissolved ions in a saturated solution. Ksp is specific to solubility and is used to determine the maximum amount of solute that can dissolve in a solvent.
• Keq: It represents the equilibrium between reactants and products in a chemical reaction. Keq is a general term for equilibrium constants and provides information about the relative concentrations (or pressures) of species at equilibrium.
2. Calculation:
• Ksp: It is calculated by multiplying the concentrations of the dissociated ions raised to the power of their stoichiometric coefficients, as derived from the balanced chemical equation for the dissolution reaction.
• Keq: It is calculated by using the concentrations (or pressures) of reactants and products at equilibrium, based on the balanced chemical equation for the reaction.
3. Nature of substances:
• Ksp: It is relevant for sparingly soluble compounds, where only a small fraction of the compound dissolves to form ions in the solvent.
• Keq: It applies to any chemical reaction, whether it is between gases, liquids, or aqueous solutions.
4. Physical interpretation:
• Ksp: It provides information about the maximum amount of solute that can dissolve in a solvent. Higher Ksp values indicate greater solubility.
• Keq: It gives insights into the relative concentrations (or pressures) of reactants and products at equilibrium, indicating the extent to which a reaction proceeds towards the products or reactants.
5. Factors affecting the constants:
• Ksp: It is influenced by temperature and the presence of common ions, which can affect the solubility of a compound.
• Keq: It is affected by temperature and pressure (for gaseous reactions) but is independent of the presence of common ions.
6. Representation of equilibrium:
• Ksp: It represents the state of equilibrium between the solute compound and its dissolved ions in a saturated solution.
• Keq: It describes the equilibrium state of a chemical reaction, indicating the balance between the concentrations (or pressures) of reactants and products at equilibrium.
Ksp is specific to solubility and describes the equilibrium between a sparingly soluble compound and its dissolved ions, while Keq is a general equilibrium constant that represents the equilibrium state of any chemical reaction. Ksp focuses on solubility and the maximum amount of solute that can dissolve, while Keq provides information about the relative concentrations (or pressures) of reactants and products at equilibrium.
Comparison chart
Sure! Here’s a comparison chart highlighting the differences between Ksp and Keq:
| Ksp (Solubility Product Constant) | Keq (Equilibrium Constant) |
|---|---|
| Describes the equilibrium between a sparingly soluble compound and its dissolved ions in a saturated solution | Represents the equilibrium between reactants and products in a chemical reaction |
| Multiplying the concentrations of dissociated ions raised to the power of their stoichiometric coefficients | Using the concentrations (or pressures) of reactants and products at equilibrium based on the balanced chemical equation |
| Relevant for sparingly soluble compounds | Applicable to any chemical reaction |
| The maximum amount of solute that can dissolve in a solvent, higher Ksp values indicate greater solubility | Relative concentrations (or pressures) of reactants and products at equilibrium, indicate the extent of the reaction |
| Temperature, presence of common ions | Temperature, pressure (for gaseous reactions), catalysts |
| Equilibrium between the solute compound and its dissolved ions in a saturated solution | The equilibrium state of a chemical reaction, the balance between concentrations (or pressures) of reactants and products |
| Does not indicate the direction of the reaction, only provides solubility information | Indicates the direction of the reaction based on the value compared to 1, favors products (Keq > 1), balanced (Keq = 1), favors reactants (Keq < 1) |
This chart summarizes the key differences between Ksp and Keq, highlighting their definitions, calculations, nature of substances, physical interpretations, factors affecting the constants, representations of equilibrium, and their relationship to the direction of the reaction.
Factors Affecting Ksp and Keq
Factors affecting Ksp (Solubility Product Constant):
1. Temperature: The solubility of a compound, and thus its Ksp value, is often temperature-dependent. In general, an increase in temperature leads to an increase in solubility and a higher Ksp value for most compounds.
2. Common ions: The presence of common ions in a solution can affect the solubility and Ksp value of a compound. Common particles are particles that are as of now displayed within the arrangement due to the nearness of other compounds. When the concentration of a common ion is increased, it can suppress the solubility of a compound and decrease its Ksp value.
Factors affecting Keq (Equilibrium Constant):
1. Temperature: The equilibrium constant, Keq, is strongly influenced by temperature. Changing the temperature of a reaction can alter the value of Keq. For some reactions, an increase in temperature favors the formation of products, resulting in a larger Keq value, while for other reactions, the reverse may be true.
2. Pressure (for gaseous reactions): If a reaction involves gaseous species, changes in pressure can affect the equilibrium constant. According to Le Chatelier’s principle, an increase in pressure tends to shift the equilibrium toward the side with fewer moles of gas, resulting in a change in the Keq value.
3. Catalysts: Catalysts do not affect the value of Keq but can increase the rate at which the equilibrium is reached. They provide an alternative reaction pathway with lower activation energy, allowing the system to reach equilibrium more quickly.
It’s important to note that both Ksp and Keq are influenced by temperature, but only Keq is affected by pressure and the presence of catalysts. Additionally, Ksp can be affected by the presence of common ions, while Keq is not directly impacted by this factor.
Conclusion
Understanding the differences between Ksp and Keq is vital for grasping the fundamental concepts of chemical equilibrium and solubility. While Ksp is a measure of solubility and describes the maximum amount of solute that can dissolve in a solvent, Keq is a measure of the extent to which a chemical reaction reaches equilibrium. The calculations, applications, and interpretation of these values differ significantly. By comprehending Ksp and Keq these contrasts, chemists can pick up profitable experiences in the behavior of chemical frameworks and make forecasts around the result of responses.