The method of disinfection chosen is crucial to ensuring the security and quality of the water supply. Two commonly used methods for disinfection are chlorination and ozonation. While both techniques aim to eliminate harmful microorganisms, they differ in their approach and effectiveness. We will look at the main distinctions between ozonation and chlorination as well as their benefits and drawbacks and their use for water-treatment processes.
Definition of Chlorination and Ozonation
Chlorination: Chlorination is a treatment procedure that involves adding chlorine and other compounds to treat water. It is among the most widely used methods for water treatment all over the world. The chlorine gas and sodium hypochlorite or calcium hypochlorite, are all commonly employed in chlorination processes. Chlorine reacts with microorganisms in water, including bacteria as well as protozoa, viruses, and protozoa, degrading the cell structure and inhibiting their reproduction. This process helps eliminate harmful pathogens and ensures the safety of drinking water.
Ozonation: Ozonation is a treatment method that makes use of the ozone (O3) which is an extremely reactive form of oxygen to purify and disinfect water. Ozone is a powerful oxidant that effectively kills microorganisms and removes contaminants in water. In the process of ozonation, ozone gas is produced and injected into water, which is where it interacts with organic substances such as bacteria, viruses, and other contaminants. This reaction breaks down the contaminants and destroys their molecular structures, rendering them harmless. Ozonation is particularly effective in removing taste and odor issues caused by organic compounds in water.
Chlorination involves the use of chlorine or chlorine compounds to disinfect water, while ozonation utilizes ozone gas for water disinfection and purification. Both methods have their unique characteristics and applications in water treatment processes.
Importance of disinfection in water treatment
Infection is a key factor in water treatment due to a variety of significant reasons:
1. Protection against Microorganisms: Disinfection focuses on getting rid of or removing harmful microorganisms, like bacteria as well as protozoa and viruses which could cause water-borne illnesses. These pathogens can trigger diseases like cholera, diarrhea, or typhoid, as well as Hepatitis A. The process ensures that the water treated is safe to drink and decreases the chance of contracting waterborne illnesses.
2. Public Health Protection: Access to clean and safe drinking water is vital for maintaining public health. Disinfection is an essential step in the process of water treatment which helps safeguard the well-being of communities by stopping the spread of waterborne illnesses. It acts as a barrier against potential health hazards associated with contaminated water sources.
3. Waterborne Disease Outbreak Prevention: Disinfection is essential in preventing outbreaks of waterborne diseases, particularly in areas with inadequate sanitation infrastructure or during emergencies and natural disasters. Disinfection provides an extra layer of protection against microbial contamination and helps safeguard public health during such critical situations.
4. Pathogen Control in Distribution Systems: Even after initial treatment, water can come into contact with microorganisms as it travels through distribution systems. Disinfection ensures that any pathogens introduced during distribution are eliminated, preventing the regrowth of bacteria and other microorganisms that could compromise water quality before it reach consumers.
5. Reducing Recreational Water Risks: Disinfection is crucial not only for drinking water but also for recreational water sources such as swimming pools, spas, and recreational water parks. Proper disinfection helps control the growth of bacteria and other microorganisms, reducing the risk of recreational waterborne illnesses and ensuring the safety of individuals using these facilities.
6. Protection against Emerging Contaminants: Disinfection can also help inactivate or remove emerging contaminants, such as certain pharmaceuticals, pesticides, and industrial chemicals, that may be present in water sources. While disinfection might not be effective against all forms of contaminants that are emerging, It may offer an additional layer of protection when combined with other filtration procedures.
Disinfection is an essential factor in water treatment because it assures the quality and safety of drinking water. It also protects the public’s health, helps prevent outbreaks of waterborne illnesses, and decreases the risks of microbial contamination that can occur in drinking water as well as water used for recreation.
What is Chlorination?
Chlorination is a water treatment method that requires using chlorine, or other compounds to treat water. It is among the most commonly used methods to disinfect water in the world. It is a common practice to use sodium hypochlorite, chlorine gas, or calcium hypochlorite are all commonly employed in chlorination processes.
Chlorination operates by introducing chlorine into the water, and it then reacts with various microorganisms such as viruses, bacteria, and protozoa. Chlorine is a potent antioxidant, disrupting the cell structure and metabolism of microorganisms, ultimately resulting in their destruction or even inactivation. This helps eliminate harmful pathogens and ensure the safety of drinking water.
Chlorination offers several advantages as a water disinfection method. It’s effective against a broad range of microorganisms. It is an effective method to prevent waterborne illnesses. Chlorine also possesses residual disinfection capabilities, meaning it can maintain its disinfecting effects as water flows through distribution systems, providing ongoing protection against microbial regrowth. Chlorination is usually regarded as an efficient and economical method to disinfect water which makes it suitable to use in massive water treatment.
Chlorination also has some drawbacks. One significant concern is the potential formation of disinfection byproducts (DBPs) when chlorine reacts with natural organic matter present in water. Some DBPs, such as trihalomethanes (THMs), can have adverse health effects. Therefore, managing and minimizing DBP formation is an important consideration in chlorination processes. Taste and odor issues can also arise due to the presence of chlorine or its byproducts in water.
Chlorination is an extensively used water treatment technique that efficiently cleanses water and helps protect public health. It is crucial for ensuring access to safe drinking water, particularly in municipal water supplies, but careful attention must be given to DBP control and managing its potential side effects.
Types of chlorination methods
There are several types of chlorination methods used in water treatment processes. The method of choice is based on factors like the application in question as well as the water quality, infrastructure, and regulatory requirements.
Here are some common types of chlorination methods:
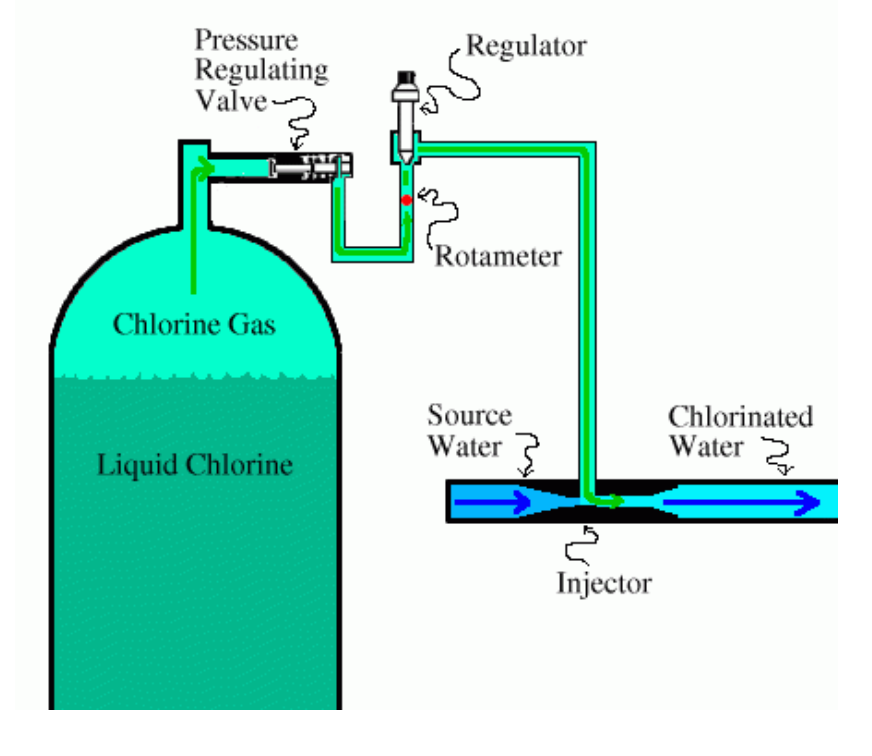
1. Chlorine Gas: Chlorine gas (Cl2) is a widely used chlorination method. It is typically introduced into the water through gas injectors or vacuum regulators. Chlorine gas is extremely efficient in disinfecting since it reacts rapidly with water and forms hypochlorous acid. It is a powerful antioxidant that kills microorganisms. The use of chlorine gas requires proper handling and safety precautions due to its toxic nature.
2. Sodium Hypochlorite: Sodium hypochlorite (NaOCl) is a chlorine compound commonly used for chlorination. It is sold in liquid forms and is commonly employed as a disinfectant in household bleach. Sodium hypochlorite is easy to handle and widely used in small-scale applications, such as residential swimming pools or small water treatment systems. It can be added directly to water or generated on-site using electrolytic systems.
3. Calcium Hypochlorite: Calcium hypochlorite (Ca(ClO)2) is another chlorine compound used for chlorination. It is typically available as a white granular solid or tablet form. Calcium hypochlorite can be found to treat water in large-scale facilities like municipal water treatment facilities or bigger swimming pools. It is more durable than sodium hypochlorite liquid and can be kept for extended durations. It is typically dissolved in water to make an acidic solution before being added to the water to be processed.
4. Electrochlorination: Electrochlorination is a chlorination method that involves using an electrolytic process to generate chlorine on-site. In this method, a low concentration of saltwater (brine) is electrolyzed, producing chlorine gas, sodium hydroxide, and hydrogen gas. The chlorine gas produced is then dissolved in water to create a chlorinated solution. Electrochlorination systems are widely employed in a variety of applications, such as plants for water treatment, treatment of waste as well as industrial processes.
5. Chlorine Dioxide: Although not strictly a chlorination method, chlorine dioxide (ClO2) is worth mentioning as it is used as a disinfectant in water treatment. Chlorine dioxide is an effective antioxidant with a wide range of antimicrobial properties. It can be generated on-site using chemicals such as sodium chlorite (NaClO2) and an acid. Chlorine dioxide is a powerful agent against viruses, bacteria, and some protozoa. It is often used for disinfection in situations where taste and odor control are critical, such as in treating water with high levels of organic matter.
These are some of the common types of chlorination methods employed in water treatment. The choice of method depends on various factors and water treatment professionals consider factors such as effectiveness, safety, cost, ease of handling, and specific treatment requirements when selecting the appropriate chlorination method for a particular application.
Chlorine gas
Chlorine gas (Cl2) is an extremely reactive and toxic gas widely employed as a disinfectant in the process of water purification. It is a green-yellow gas that has a distinct smell similar to the odor of bleach.
Chlorine gas is commonly used because of its powerful chemical oxidizing and disinfecting capabilities. When chlorine gas is dissolved in water, it forms hypochlorous acid (HOCl) and hypochlorite ions (OCl-). They are capable of eliminating a variety of microorganisms like viruses, bacteria, and protozoa, by altering their cell structures and metabolic processes.
Chlorine gas is typically introduced into the water treatment system through gas injectors or vacuum regulators. The gas is carefully controlled and added in precise dosages to ensure effective disinfection while minimizing the formation of disinfection byproducts (DBPs). The concentration of chlorine gas used in water treatment is typically low and closely monitored to maintain safe levels.
It is important to note that handling chlorine gas requires proper safety precautions due to its hazardous nature. Chlorine gas may cause severe eye and respiratory irritations. It can be toxic when inhaled in high concentrations. The water treatment plants that utilize chlorine gas must follow strict safety standards, including proper storage, handling as well as ventilation methods to protect workers and the environment around them.
Despite the risk of harm that is associated with the use of chlorine gas, it’s still a popular and effective method for disinfecting water treatment because of its broad-spectrum disinfection abilities, residual disinfection properties, as well as cost-effectiveness. Efforts are made to minimize the use of chlorine gas or find alternatives where possible to address safety concerns and reduce the formation of disinfection byproducts.
Sodium hypochlorite
Hypochlorite sodium (NaOCl) is an ingredient in chlorine that is commonly employed as a disinfectant in water treatment. It is a clear, yellowish liquid with a strong chlorine odor. Sodium hypochlorite is commonly known as liquid bleach. It’s widely accessible for a range of uses that include household cleaning, water treatment, and maintenance of swimming pools.
Sodium hypochlorite can be used as the source of chlorine used for disinfection. It’s efficient against a variety of microorganisms including viruses, bacteria, and protozoa. The process operates by releasing hypochlorous acids (HOCl) as well as hypochlorite Ions (OCl-) upon coming into contact with water. These substances act as powerful antioxidants, disrupting the metabolism and cellular structures of microorganisms. This results in their destruction or deactivation.
The sodium hypochlorite compound is fairly simple to work with and is commonly used in small-scale settings like household disinfection, a small-scale system of water treatment, and residential swimming pools. It is typically available in varying concentrations, with household bleach containing around 5-6% sodium hypochlorite. Commercially-grade sodium hypochlorite solutions that have higher concentrations can also be utilized in larger-scale water treatment facilities.
In the case of sodium hypochlorite being used for water disinfection, you must be essential to think about the dosage as well as contact time and pH of the water to ensure that the disinfection process is effective. The concentration of sodium hypochlorite required for disinfection purposes depends on the water quality, target microorganisms, and specific treatment requirements.
It is important to note that the application of sodium hypochlorite may cause the formation DBPs from disinfection (DBPs) when it interacts with organic matter found in water. Proper dosage and control measures are necessary to minimize the formation of DBPs, such as trihalomethanes (THMs), which can have potential health implications.
Sodium hypochlorite can be a widely utilized and easily accessible disinfectant for water treatment. It offers effective disinfection capabilities against microorganisms and is relatively easy to handle. Safety precautions that are appropriate, such as the proper handling, storage, as well as dosage controls, must be taken to ensure safe and efficient usage.
What is Ozonation?
Ozonation is a water treatment process that utilizes ozone (O3) to disinfect and purify water. Ozone is a potent antioxidant and is a highly reacting version of oxygen. It is generated by passing dry air or pure oxygen through an ozone generator that applies high-voltage electrical discharges.
During the ozonation process, ozone gas is introduced into water, where it undergoes chemical reactions with various contaminants and microorganisms. Ozone is a potent disinfectant, destroying bacteria and viruses, algae, and other pathogens by destroying their cell walls and disrupting their metabolism processes. It also oxidizes and breaks down organic compounds, removing tastes, odors, and color caused by natural organic matter or certain pollutants.
Ozone offers several advantages as a water treatment method. Firstly, it has a high disinfection efficacy and a broad spectrum of activity against microorganisms. It is particularly effective against some chlorine-resistant pathogens and provides superior disinfection performance compared to traditional disinfectants like chlorine. Secondly, ozone does not produce significant levels of disinfection byproducts (DBPs) because it decomposes into oxygen after reacting with contaminants. This is a significant advantage in terms of water quality and safety.
Ozonation can enhance the overall water treatment process by improving the removal of certain pollutants, such as organic chemicals, pesticides, and taste- and odor-causing compounds. It can also aid in the coagulation and flocculation processes by assisting in the removal of suspended solids and improving the efficiency of downstream filtration.
Ozonation also has some limitations and considerations. One major drawback is that ozone does not provide residual disinfection in the distribution system, unlike chlorine. Therefore, additional disinfection methods may be required to maintain a residual disinfectant and prevent microbial regrowth. Ozone treatment also requires careful monitoring and control, as the dosage and contact time must be optimized to achieve the desired disinfection and oxidation goals. Furthermore, ozonation systems typically have higher capital and operational costs compared to traditional disinfection methods.

Ozonation is a powerful water treatment process that offers effective disinfection, oxidation, and removal of contaminants. Its advantages in terms of disinfection efficacy, minimal formation of disinfection byproducts, and removal of taste and odor issues make it a popular choice in various water treatment applications.
Advantages of ozonation
Ozonation offers several advantages as a water treatment method.
Here are some of the key advantages of ozonation:
1. Strong Disinfection: Ozone is a powerful disinfectant that effectively kills a wide range of microorganisms, including bacteria, viruses, and protozoa. It has a higher disinfection efficacy compared to traditional disinfectants like chlorine. Ozone can even be effective against chlorine-resistant pathogens, making it a valuable tool for ensuring water safety.
2. Oxidation of Contaminants: Ozone is a strong oxidizing agent that can break down and oxidize various contaminants in water. It effectively removes organic compounds, such as natural organic matter, taste and odor-causing compounds, and certain pollutants like pesticides and pharmaceuticals. This helps improve water quality and reduces the presence of unwanted substances.
3. Minimal Disinfection Byproducts: Unlike chlorine, ozone does not produce significant levels of disinfection byproducts (DBPs). DBPs, such as trihalomethanes (THMs), can have potential health implications. Ozone decomposes into oxygen after reacting with contaminants, leaving no residual disinfectant or harmful byproducts, making it a favorable choice for water treatment.
4. No Taste or Odor Impact: Ozone treatment effectively eliminates taste and odor issues caused by organic compounds in water. It oxidizes and breaks down these compounds, resulting in improved taste and odor of the treated water. This is especially beneficial for applications where high-quality water with no off-putting taste or odor is required.
5. Enhanced Coagulation and Filtration: Ozonation can improve the coagulation and flocculation processes in water treatment. Ozone aids in the removal of suspended solids by assisting in the agglomeration of particles, making them easier to remove through filtration. This can enhance the efficiency of downstream filtration processes, resulting in improved water clarity and reduced turbidity.
6. Environmental Benefits: Ozone is a sustainable and environmentally friendly disinfection method. It does not contribute to the formation of persistent disinfection byproducts in water or produce harmful residuals. Additionally, ozone treatment requires less contact time compared to other disinfectants, reducing the overall treatment time and energy consumption.
It’s worth noting that while ozonation offers numerous advantages, it also has some considerations. Ozone treatment systems typically have higher capital and operational costs compared to traditional disinfection methods, requiring specialized equipment and expertise. The dosage and control of ozone must be carefully monitored to ensure optimal disinfection and oxidation results. Additionally, ozone does not provide residual disinfection, so other disinfection methods may be necessary to maintain a disinfectant residual in the distribution system.
Ozonation is a powerful water treatment process that offers effective disinfection, oxidation of contaminants, and removal of taste and odor issues. Its advantages in terms of disinfection efficacy, minimal disinfection byproducts, and environmental sustainability make it a valuable option for various water treatment applications.
Powerful disinfection capability
One of the significant advantages of ozonation is its powerful disinfection capability. Ozone is a highly effective disinfectant, capable of killing a wide range of microorganisms, including bacteria, viruses, and protozoa.
Here are some key reasons why ozonation is considered to have a powerful disinfection capability:
1. Broad-Spectrum Disinfection: Ozone is a broad-spectrum disinfectant, meaning it can effectively target and destroy a wide range of microorganisms. It is particularly effective against chlorine-resistant pathogens, providing an additional layer of protection against waterborne diseases.
2. Rapid and Immediate Action: Ozone acts quickly upon contact with microorganisms. It has a faster disinfection rate compared to other disinfectants, such as chlorine. This rapid action allows for more efficient and timely treatment of water, ensuring effective microbial control.
3. Effective Against Biofilms: Biofilms are slimy layers of microorganisms that can form on surfaces in water distribution systems or treatment equipment. They can act as a protective barrier, making it difficult for traditional disinfectants to penetrate and eliminate microorganisms. Ozone, with its strong oxidation potential, can effectively break down biofilms and destroy microorganisms within them.
4. High Oxidation Potential: Ozone is a powerful oxidizing agent, meaning it can readily react with and oxidize various organic and inorganic compounds. It disrupts the cellular structures and metabolic processes of microorganisms, leading to their destruction or inactivation. This high oxidation potential contributes to its strong disinfection capability.
5. No Residual Disinfectant: While the absence of a residual disinfectant can be considered a disadvantage in terms of maintaining disinfection in the distribution system, it can also be an advantage in certain applications. For example, in water used for food processing or certain industrial processes, the absence of a residual disinfectant like chlorine can be desirable to avoid any potential taste, odor, or chemical interference.
It is important to note that the disinfection capability of ozonation can be affected by various factors, including ozone dosage, contact time, water quality, and temperature. Proper monitoring and control of these factors are crucial to ensure optimal disinfection results.
Ozonation’s powerful disinfection capability makes it a valuable tool in water treatment. Its effectiveness against a wide range of microorganisms, rapid action, and oxidation potential contribute to its ability to provide safe and disinfected water.
No formation of significant disinfection byproducts
Another advantage of ozonation is that it does not produce significant levels of disinfection byproducts (DBPs). Disinfection byproducts are compounds that form when disinfectants react with organic matter present in water. Some common disinfection byproducts include trihalomethanes (THMs), haloacetic acids (HAAs), and chlorite.
Ozone, being a strong oxidizing agent, does not form the same types of disinfection byproducts as chlorination, for example. When ozone reacts with organic matter in water, it breaks down and oxidizes the compounds without producing high levels of DBPs. This is in contrast to chlorine, which can react with organic matter to form potentially harmful byproducts.
By minimizing the formation of disinfection byproducts, ozonation helps to improve the overall water quality and reduces potential health risks associated with consuming water containing elevated levels of DBPs. This advantage is particularly important in situations where there are strict regulatory limits on disinfection byproducts or when the taste, odor, and aesthetic quality of the water are of concern.
It is important to remember that even though ozonation generates fewer disinfection byproducts than other disinfection methods but it is nevertheless crucial to keep track of and manage the water treatment process to reduce the risk of forming byproducts. The dose of ozone, contact times as well as water quality, and pH must be properly monitored to ensure the effectiveness of the process of disinfection and reduce the creation of any residual byproducts that might be present.
The reduced formation of disinfection byproducts is an important advantage of ozonation, promoting healthier and aesthetically pleasing water for various applications.
Comparison Chart
Here is a comparison chart highlighting the key differences between chlorination and ozonation:
| Aspect | Chlorination | Ozonation |
|---|---|---|
| Chemical Used | Chlorine-based compounds (chlorine gas or sodium hypochlorite) | Ozone gas (O3) |
| Disinfection Mechanism | Forms hypochlorous acid (HOCl) and hypochlorite ions (OCl-) to disrupt microbial structures and processes | Oxidizes and damages microbial cell walls and metabolic processes |
| Disinfection Byproducts | Can form disinfection byproducts (DBPs), such as trihalomethanes (THMs) and haloacetic acids (HAAs) | Produces fewer disinfection byproducts |
| Residual Disinfectant | Leaves a residual disinfectant in water for ongoing protection against microbial regrowth | Does not provide a residual disinfectant |
| Treatment Efficiency | Effective in providing disinfection and microbial control | Highly effective in disinfection and oxidation, improving water quality |
| Operational Considerations | Relatively straightforward to operate and requires less complex equipment | Requires specialized equipment and higher capital/operational costs |
| Environmental Impact | This can result in the formation of potentially harmful disinfection byproducts | Produces minimal disinfection byproducts |
| Applications | Widely used in various water treatment systems | Used in specific applications, often for advanced disinfection and improving water quality |
Conclusion
Chlorination and ozonation are two prominent methods for water disinfection, each with its own set of advantages and limitations. Chlorination provides effective disinfection at a lower cost but may lead to the formation of disinfection byproducts and affect taste and odor.
On the other hand, ozonation offers powerful disinfection without the formation of harmful byproducts but at a higher cost. The choice between the two methods depends on various factors, including water quality, target pathogens, and treatment goals. Water treatment professionals should carefully evaluate these factors to determine the most suitable disinfection approach for their specific needs.
