Neutralization and Titration are two processes in the chemical industry that are used frequently in industrial and scientific laboratories. They are frequently mistaken for one another and are sometimes used in conjunction.
There are some important differences between these two methods that make them distinct. Understanding these differences is vital in determining which one is most suitable for a specific chemical analysis.
Importance of understanding the difference between titration and neutralization
Understanding the distinction between neutralization and titration is vital for many reasons:
1. Conceptual clarity: Titration and neutralization are two distinct concepts in chemistry. Understanding the differences between them helps prevent confusion and encourages conceptual clarity. It helps learners discern between these two processes and use the correct techniques in real-world situations.
2. Experimental design: knowing the differences between neutralization and titration is essential for the design of experiments. It assists scientists and researchers in selecting the best method, based on the purposes of their research. If the aim is determining the level of an unidentified solution, titration is the most appropriate method, but when the goal is to neutralize an acidic or basic substance neutralization reactions are better suited.
3. Titration in analytical: Chemistry is a fundamental method in the field of analytical chemistry that is used to determine the level of substance present in the solution. With a thorough understanding of titration, researchers can measure accurately unknown concentrations and guarantee quality control in a range of industries like food and drink, pharmaceuticals environmental testing, and so on. Knowing the difference between neutralization and titration avoids incorrect interpretations or laboratory setups in the practice of analytical chemistry.
4. Acid-base chemistry: Acid-base reactions are a fundamental part of chemical chemistry and are used in a variety of ways in daily life. Knowing the difference between neutralization and titration aids in gaining a better understanding what occurs in the acid base reactions. It helps students grasp concepts such as indicators, ph such as equivalence points along the creation of salts that are essential to acid-base chemical reactions.
5. Practical applications: the understanding of titration and neutralization has useful applications across a variety of disciplines. For instance, in the medical field understanding titration is essential in determining the right dosage of medicines. In the field of environmental science, an understanding of neutralization reactions aids in the treatment of basic or acidic wastewater before its release into the environment. By understanding the difference between neutralization and titration experts in these fields can make informed choices and perform their tasks efficiently.
Understanding the distinction between neutralization and titration is crucial for a clear understanding of concepts, experiments as well as an understanding of acid-base reactions, and their practical application in a variety of areas of science. It allows accurate analysis, supports the proper experiments, and facilitates the use of informed decisions in industrial and scientific processes.
Titration
Titration is a method that is used in analytical chemistry to determine the amount of a substance in the solution. It is adding a chemical reagent (known as the titrant) to a solution that contains the substance of interest (the substance of importance) till the reaction that occurs between the titrant and the analyte is completed. At what point the reaction is considered to be complete known as the equivalence point.
Titration’s purpose is to precisely determine the amount of an unknown chemical by comparison with an established standard solution with a known concentration. This method allows for exact quantitative analysis of chemicals that are used in a variety of industries, such as environmental monitoring, pharmaceuticals, food and beverages, and chemical manufacturing.
The process of Titration typically consists of one of the steps below:
- Selection of indicators: Indicators are substances that show a distinct color change after the reaction has reached the point of equivalence. The selection of the indicator will depend on the kind of reaction that is being carried out (e.G. acid-base or Redox).
- Preparation of analyte and titrant solutions: The titrant solution is made by dissolving a predetermined amount of the reagent in an appropriate solvent. Analytes are made by dissolving an unknown ingredient in a solution, or by diluting the sample of the substance.
- The determination of the equivalence level: The titrant will be added to the solution of the analyte slowly, usually with a burette. This is done while keeping track of the process’s progress. This can be accomplished visually by watching the change in color of the indicator or employing instruments like a pH gauge or an equivalence potentiometer.
- The unknown concentration is calculated: the quantity of titrant that is required to get to the point of equivalence is determined. With the knowledge of the level of titrant, as well as the amount used for the measurement, the amount in the substance that is unknown may be calculated by stoichiometry or an equation for balanced chemicals.
There are various types of titrations based on the type of reaction being investigated. The most popular kind is acid-base titration where an acid can react with a base, or in reverse to determine its concentration. Redox titrations are based on oxidation-reduction reactions, while precipitation titrations are the result of the precipitation of a substance.
Titration is an extensively used method due to its accuracy as well as its versatility and application across various industries. It permits the precise measurement of concentrations as well as quality control in manufacturing processes the detection of impurities and the analysis of complex mix-ups.
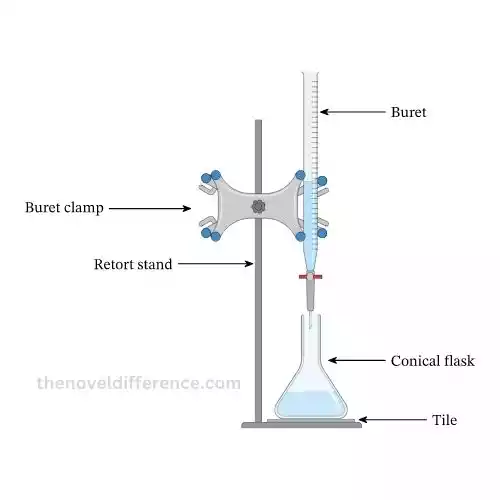
Procedure of titration
Titration is a method to measure the amount of a substance in a solution. Here’s a step-by-step guide:
1. Set Up Equipment:
- Get the tools you need: a burette, pipette, flask, beaker, and any chemicals you’ll use.
- Clean the equipment to remove any dirt or impurities.
- Arrange everything: attach the burette to a stand and put the flask under it.
2. Prepare Solutions:
- Make the titrant solution: mix a known concentration chemical with a suitable liquid.
- Create the analyte solution: mix the substance you want to measure with a liquid. If it’s too concentrated, dilute it.
3. Start Titration:
- Fill the burette with the titrant solution and note its starting volume.
- Measure a precise amount of analyte solution in the flask using a pipette.
- If needed, add a few drops of an indicator that changes color during the reaction.
4. Titrate:
- Slowly add the titrant solution from the burette to the flask while gently swirling the flask.
- Be careful near the endpoint to avoid adding too much titrant. This helps get precise results.
- Look for changes like a color shift or the formation of solid particles. Note the volume of titrant used.
5. Find the Endpoint:
- If you can’t see a color change, use special tools like a pH meter to find the endpoint.
- The endpoint means the reaction between the analyte and titrant is finished. They’ve reacted in the right amounts.
6. Calculate:
- Use the volume of titrant at the endpoint and the known concentration to calculate the analyte’s amount or concentration.
- Consider any dilutions if you used them.
7. Repeat and Average:
- Do the titration a few times to make sure your results are accurate.
- Find the average of your results to minimize errors.
Remember, the steps can change depending on the type of titration and the substances you’re working with. Always be precise and careful when measuring and recording volumes for reliable results.
Types of Titration
Titration is a common laboratory technique used to determine the concentration or characteristics of specific substances in a solution. There are various types of titration methods, each tailored for specific purposes and substances.
Here, we’ll break down these titration types in easy-to-understand terms:
- Acid-Base Titration: This method involves measuring the concentration of acids or bases in a solution. It’s all about neutralizing acids with bases or vice versa. To know when the reaction is complete, we often use indicators like phenolphthalein or methyl orange, which change color when the acid and base have mixed in the right proportions.
- Redox Titration: In redox titration, we measure the concentration of substances that can either gain or lose electrons. We use chemicals like potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7) as indicators. They help us determine the amount of these electron-exchanging substances in a solution.
- Precipitation Titration: This titration relies on forming solid particles (precipitates) during a chemical reaction. It’s used to determine the concentration of ions that can make insoluble salts. We can see when the reaction is done either by the visible formation of precipitates or by checking changes in conductivity.
- Complexometric Titration: Complexometric titration deals with the interaction between metal ions and special chemicals called complexing agents or ligands. We use it to find the concentration of metal ions or to identify which metal ions are in a solution. A famous complexing agent is ethylenediaminetetraacetic acid (EDTA).
- Non-Aqueous Titration: Sometimes, water isn’t the right solvent to use. In non-aqueous titration, we perform titration in solvents other than water, like organic solvents. This is handy when the substances we’re analyzing don’t dissolve well in water or when water could mess up the reaction.
- Coulometric Titration: Coulometric titration is a precise method for measuring tiny amounts of a substance. It works by figuring out how much electricity is needed to complete a chemical reaction.
- Back Titration: When a direct titration isn’t suitable, or the reaction is slow or incomplete, we use back titration. It involves reacting the analyte with an excess of a known reagent and then titrating the leftover reagent with another solution of known concentration.
These are just a few examples of the types of titration you might encounter in analytical chemistry. Each type has a unique purpose and method, making them valuable tools in various industries and research fields.
Neutralization
Neutralization is like chemical teamwork between acids and bases that creates salt and water. Imagine it as a friendly exchange of protons (H+) from the acid to hydroxide ions (OH-) from the base.
Here’s the basic idea: Acid + base → salt + water
What’s cool about neutralization:
- Acid-Base Party: Neutralization is when acids and bases get together. Acids share protons, and bases receive them. The result? Water and salt.
- Salt Creation: As a bonus, we get salt! It’s like a mix of ions from the acid and base. The exact salt depends on the types of acid and base used.
- Balancing pH: Neutralization turns things neutral. The pH (a measure of acidity) goes close to 7, which is neither too acidic nor too basic.
- Recipe Matters: The reaction follows a recipe based on the balanced equation. It stops when there’s just the right amount of acid and base, making everything neutral.
- Heat Show: Neutralization often heats things up. This heat can be used in practical ways, like self-heating food or hand warmers.
Where you see neutralization:
- Tummy Troubles: It’s used in antacid medicines to calm stomach acid, helping with heartburn and acid reflux.
- Eco-Friendly Cleanup: Wastewater treatment uses neutralization to make acidic or basic wastewater less harmful before it goes into the environment.
- Chemistry Magic: In various industries, neutralization controls pH and improves chemical reactions. It’s handy in making stuff, like medicines and materials.
Understanding neutralization helps keep things balanced, controls pH, and fixes acid-base issues. Scientists and engineers use it to create solutions for lots of practical things.
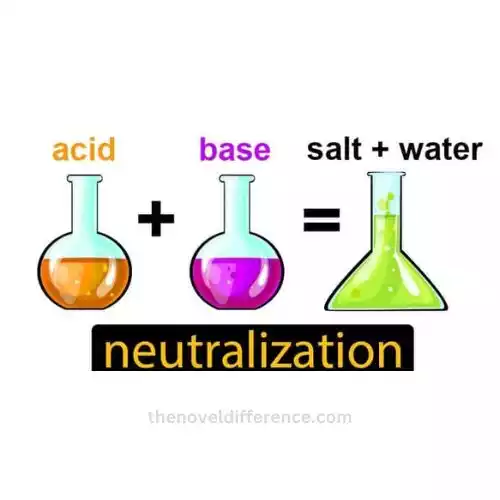
The Chemical Reaction in Neutralization
When we talk about neutralization, we’re describing a chemical reaction that happens when an acid and a base come together. This reaction results in the creation of salt and water.
Let’s break it down in a simple way:
Imagine it as a meeting between an acid and a base. The acid gives away a tiny particle called a proton (it’s like a friendly gesture), and the base happily accepts it. This exchange of protons is what makes the acid and base lose their unique, strong characteristics and turn into water and salt, which are more neutral.
To write it as an equation, it looks like this: Acid + base → salt + water.
The exact equation you use depends on which acid and base are getting together. Let’s look at a few examples:
- Imagine hydrochloric acid (HCl) meeting sodium hydroxide (NaOH). They shake hands like this: HCl + NaOH → NaCl + H2O. HCl gives its proton (H+) to the hydroxide ion (OH-) from NaOH, and they create sodium chloride (NaCl) and water (H2O).
- Now, picture sulfuric acid (H2SO4) having a chat with calcium hydroxide (Ca(OH)2). They create a friendly atmosphere like this: H2SO4 + Ca(OH)2 → CaSO4 + 2H2O. H2SO4 combines with Ca(OH)2, resulting in calcium sulfate (CaSO4) and two molecules of water (2H2O).
- Lastly, think of acetic acid (CH3COOH) mingling with ammonia (NH3). They form a partnership like this: CH3COOH + NH3 → NH4CH3COO. CH3COOH joins NH3, creating ammonium acetate (NH4CH3COO).
These examples show the basic idea of neutralization: the acid gives a proton (H+), the base takes it, and it turns into salt and water. The specific salt you get depends on which acid and base are involved.
Remember, not all neutralization reactions create salts that dissolve in water. Some reactions result in insoluble salts, which means the salt can’t stay in the water and settles down at the bottom.
Factors That Affect Neutralization
Now, let’s talk about what can influence this neutralization process. A few things can change how fast and how complete the reaction is:
- Concentration of Acid and Base: If there are lots of acid and base particles in the mix, the reaction happens faster. It’s like having more people at a party; there’s a better chance they’ll interact.
- Strength of Acid and Base: Some acids and bases are more willing to give or take protons. Strong ones do this very easily, while weaker ones need more encouragement. Stronger acids and bases lead to quicker neutralization.
- Temperature: Changing the temperature can speed up or slow down the reaction. Higher temperatures make particles move faster, so they bump into each other more often, causing the reaction to happen more quickly. Extremely hot or cold temperatures can also mess things up, though.
- Surface Area: If a solid acid or base is involved, breaking it into smaller pieces or making it into a fine powder increases the chances for contact between the reactants, making the reaction quicker.
- Catalysts: These are like special cheerleaders for the reaction. They can make it happen faster, but they don’t get used up in the process. Not all reactions need catalysts, though.
- Stoichiometry: This is just a fancy word for the right balance. The equation tells you how much acid and base you need for a full neutralization. If you don’t have enough of one or the other, the reaction won’t be complete.
- Nature of the Acid and Base: Different acids and bases have their unique personalities. Some are more reactive or volatile, affecting how the reaction plays out.
Remember, these factors can work together in complicated ways, and adjusting them can help you control the neutralization process for specific situations. It’s like fine-tuning a recipe to get the perfect result.
Key comparison chart of titration and neutralization
Here’s a key comparison chart of titration and neutralization:
| Aspect | Titration | Neutralization |
|---|---|---|
| Definition | Titration is a laboratory technique used to determine the concentration of a substance (usually an analyte) in a solution by reacting it with a solution of known concentration (usually a titrant) until a chemical reaction is complete. | Neutralization is a type of chemical reaction where an acid and a base react to form water and a salt, resulting in the neutralization of their acidic and basic properties. |
| Purpose | The primary purpose of titration is quantitative analysis, which involves measuring the unknown concentration of a substance. | Neutralization is a chemical reaction that occurs when an acid and a base come into contact, resulting in the formation of water and salt. It is often used to neutralize acidic or basic solutions. |
| Reaction | Titration typically involves a chemical reaction between the analyte and titrant, which is chosen to react completely with the analyte. The reaction is usually not limited to acid-base reactions and can involve various reactions based on the specific analysis. | Neutralization is a specific type of chemical reaction, and it always involves the reaction of an acid and a base to produce water and salt. |
| Endpoint Detection | In titration, the endpoint of the reaction is detected using indicators, pH meters, or other methods, and it’s crucial to identify the exact point at which the reaction is complete to determine the concentration accurately. | In neutralization, the endpoint of the reaction is usually reached when all the acid and base have reacted completely, and it is generally more straightforward to detect without the need for indicators or sophisticated equipment. |
| Applications | Titration is commonly used in analytical chemistry for various purposes, such as determining the concentration of acids, bases, salts, and other analytes in solutions. | Neutralization is a fundamental chemical process and has applications in various fields, including acid-base reactions in the human body (e.g., digestion), wastewater treatment, and the preparation of specific chemical compounds. |
| Measurement Units | The results of titration are typically expressed in terms of molarity (moles per liter) or other concentration units. | The results of neutralization are often described in terms of the amount of acid or base required for the reaction to complete or the amount of salt produced. |
| Equipment | Titration requires specialized equipment, including burettes, pipettes, titration flasks, and indicators. | Neutralization can be performed with basic laboratory glassware and does not require the same level of specialized equipment as titration. |
| Types | There are various types of titrations, including acid-base titrations, redox titrations, and complexometric titrations, each with its own specific purpose and reagents. | Neutralization is a specific type of reaction and does not have variations like titrations. |
Titration is a quantitative analytical technique used to determine the concentration of a substance in a solution, while neutralization is a specific type of chemical reaction that occurs when an acid and a base combine to form water and salt, neutralizing their properties. Both have their distinct purposes and characteristics.
Similarities Between Titration and Neutralization
Titration and neutralization, although separate concepts share several commonalities that make them easier to understand:
- Acid-Base Chemistry: Both titration and neutralization are rooted in acid-base reactions. They both revolve around the interaction between an acid and a base. This interaction is either a central part of the titration process or forms the primary reaction in neutralization.
- Use of Indicators: Indicators find application in both titration and neutralization. These are substances that change color based on the pH (acidity or alkalinity) of a solution. They serve the crucial purpose of indicating the endpoint in a titration or the point of neutralization in a neutralization reaction.
- Formation of Water: In both titration and neutralization reactions, one common outcome is the creation of water as a product. In neutralization, water emerges as the acidic and basic characteristics of the reactants are nullified. In certain titration reactions, water may be generated as a byproduct of the chemical reaction between the analyte and the titrant.
- Stoichiometric Calculations: Both titration and neutralization reactions require stoichiometric calculations. Stoichiometry is the branch of chemistry that deals with the relative quantities of reactants and products in a chemical reaction. It’s employed to determine the concentration of the analyte based on the volume and concentration of the titrant used. In essence, stoichiometry is utilized to identify the balanced equation and the amounts of acid and base necessary for complete neutralization.
- Practical Applications: Titration and neutralization have a multitude of practical uses across various fields. Titration is commonly employed in analytical chemistry for quantitative analysis and quality control purposes. Neutralization, on the other hand, is used in diverse applications such as antacid consumption for alleviating acidity, wastewater treatment, and maintaining the desired pH level in chemical processes.
Despite these similarities, it’s important to distinguish between titration and neutralization based on their distinct objectives and procedures. Titration is a method employed to ascertain the concentration of a specific substance, while neutralization is a chemical reaction that involves the nullification of acidic and basic properties, resulting in the formation of water.
Conclusion
Knowing the distinctions between neutralization and titration is essential for a precise chemical analysis. While titration measures the amount of an unidentified substance, neutralization concentrates upon the reaction that occurs between an acid and an acid.
Both procedures have distinct applications and advantages in a variety of industries. In defining the differences between neutralization and titration you will be able to select the most suitable method to meet your needs for analysis.
