Micelles and colloidal particles are fascinating entities that play crucial roles in various scientific disciplines, including chemistry, physics, and biology. These microscopic structures possess unique properties and exhibit distinct behavior, making them essential subjects of study.
We delve into the captivating world of micelles and colloidal particles, exploring their characteristics, differences, and applications. So, let’s embark on this journey and uncover the disparities between these two intriguing entities.
What are Micelles?
Micelles are self-assembled structures that form in solutions, particularly in the presence of amphiphilic molecules. These amphiphiles have both hydrophilic (water-attracting) and hydrophobic (water-repelling) regions within their molecular structure.
When amphiphilic molecules are introduced into a solvent, such as water, they arrange themselves in a specific way due to their dual nature, creating micelles.
Key characteristics of micelles include:
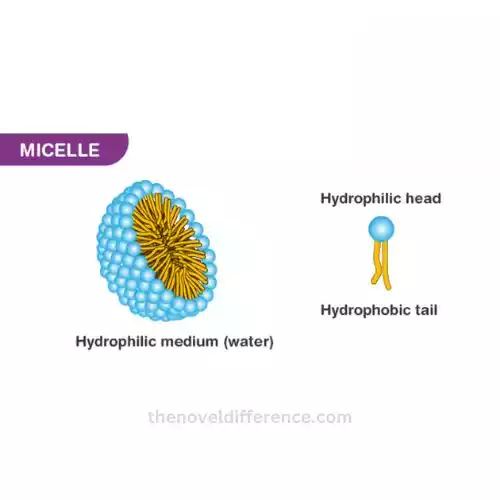
- Amphiphilic Nature: Micelles form because amphiphilic molecules, such as surfactants or lipids, have both hydrophilic and hydrophobic parts. The hydrophilic portion interacts with water, while the hydrophobic part avoids contact with water.
- Core-Shell Structure: Micelles typically have a core made up of hydrophobic tails of the amphiphilic molecules, shielded by a shell of hydrophilic heads. This core-shell structure is essential for micelle stability.
- Reduction of Surface Tension: Micelles have the ability to reduce the surface tension of a liquid, which is why they are commonly found in detergents and soaps. The hydrophobic tails aggregate in the core of the micelle, while the hydrophilic heads interact with the surrounding solvent.
- Encapsulation of Hydrophobic Substances: The hydrophobic core of micelles provides an environment where hydrophobic substances, which would not readily dissolve in water, can be solubilized and transported. This property is utilized in drug delivery, particularly for drugs that are poorly water-soluble.
Micelles have a wide range of applications in various industries, including the pharmaceutical, cosmetic, and food industries, where they are used for their ability to improve solubility, stabilize colloidal systems, and enhance the dispersion of substances with hydrophobic properties.
What are Colloidal Particles?
Colloidal particles are small particles, typically in the size range of nanometers to micrometers, that are dispersed in a continuous phase, which can be a liquid, gas, or solid. These particles are intermediate in size between individual molecules or ions and larger visible particles.
Colloidal systems consist of these finely divided particles dispersed within a dispersing medium.
Key characteristics of colloidal particles and colloidal systems include:
- Size Range: Colloidal particles are often in the size range of 1 nanometer (nm) to 1 micrometer (μm). This size range distinguishes them from molecules or ions, which are much smaller, and from larger visible particles.
- Dispersion: Colloidal particles are dispersed within a continuous phase, forming a colloidal dispersion or colloidal system. The dispersing medium can be a liquid, gas, or solid.
- Types of Colloids: There are various types of colloidal systems, including:
- Sols: Liquid colloids where solid particles are dispersed in a liquid medium.
- Emulsions: Liquid-liquid colloids where one liquid is dispersed in another liquid.
- Aerosols: Liquid or solid particles dispersed in a gas.
- Foams: Gas dispersed in a liquid or solid.
- Suspensions: Solid particles dispersed in a liquid or gas.
- Stability and Aggregation: The stability of colloidal systems is influenced by factors such as the electric charge on the particles, the presence of stabilizing agents, and the temperature. Colloidal particles can aggregate or coagulate if the conditions are not conducive to their stability.
- Brownian Motion: Colloidal particles exhibit Brownian motion, a random motion resulting from collisions with molecules of the dispersing medium. This motion is visible under a microscope and is an important characteristic of colloids.
- Tyndall Effect: When a beam of light is passed through a colloidal dispersion, the path of the light becomes visible due to scattering by the colloidal particles. This is known as the Tyndall effect and is a common method to confirm the presence of colloidal particles.
Colloidal particles and colloidal systems have numerous practical applications in various fields. For example, they are used in food and beverage production to stabilize products, in pharmaceuticals for drug delivery, in paints and coatings for pigment dispersion, and in environmental science for wastewater treatment. Understanding the properties and behavior of colloids is important for optimizing processes and developing new materials and products.
Formation and structure of Micelles and Colloidal Particles
Formation of Micelles:
Micelles are tiny structures that form when certain special molecules, called amphiphiles, are put into water. These amphiphiles have a water-loving (hydrophilic) part and a water-fearing (hydrophobic) part.
Here’s how they form:
- Dissolution: First, these special molecules dissolve in the water. They have a head that likes water and a tail that doesn’t.
- Aggregation: As more of these molecules join, their tails stick together because they don’t like water. This forms the core of the micelle.
- Hydrophilic Shell: Meanwhile, the water-loving heads stay on the outside, surrounding the core. This shell keeps everything stable.
The result is a little ball-like structure called a micelle, with the tails hidden inside and the heads sticking out. This helps them trap other molecules that don’t like water, which can be very useful in certain applications.
Formation of Colloidal Particles:
Colloidal particles can be made in different ways, depending on what kind they are.
Here are some common ways they’re made:
- Dispersion: Solid particles can be mixed into a liquid through stirring, milling, or other methods, creating a suspension of tiny particles in the liquid.
- Emulsification: Mixing two liquids that don’t usually mix can create an emulsion. For example, think of how oil and water don’t usually mix, but you can make a mixture like mayonnaise if you add an emulsifier.
- Aerosol Generation: When you spray something like perfume or use a spray can, you’re creating an aerosol. This is when tiny particles of liquid or solid are suspended in a gas.
- Chemical Synthesis: Some particles can be made in a lab by reacting chemicals together. This can be used to make really tiny particles, like those used in some medicines or electronics.
Structure of Colloidal Particles:
The structure of colloidal particles can be different depending on what they’re made of and how they’re made:
- Sols: These are when solid particles are mixed into a liquid, forming a kind of suspension.
- Emulsions: If you mix two liquids that don’t usually mix, you get emulsions, which can be things like milk or mayonnaise.
- Aerosols: These are tiny particles suspended in a gas, like what you see when you spray something from a can.
- Foams: These are like bubbles in a liquid or solid, like the froth on top of a bubble bath.
- Suspensions: When you mix solid particles into a liquid, you get a suspension, like the muddy water in a puddle.
Understanding these structures is important because it helps us know how to use them in different ways, like in medicines, food, and other everyday things.
Differences between micelles and colloidal particles
Micelles and colloidal particles are both types of dispersed systems, but they exhibit distinct differences in their formation, structure, and behavior.
Here are the key differences between micelles and colloidal particles:
1. Formation:
• Micelles: micelles are formed through the self-assembly of amphiphilic molecules in a solvent, typically water. The hydrophilic regions of the amphiphilic molecules interact with the surrounding solvent, while the hydrophobic regions aggregate in the core, forming a spherical or ellipsoidal structure.
• Colloidal particles: colloidal particles can have various origins and formation mechanisms. They can be formed by dispersion or condensation processes, such as the aggregation of nanoparticles, the emulsification of immiscible liquids, or the formation of gas bubbles in a liquid.
2. Size range:
• Micelles: micelles are typically on the smaller end of the colloidal size range, with diameters typically ranging from 1 to 100 nanometers.
• Colloidal particles: colloidal particles encompass a broader size range, generally ranging from 1 nanometer to 1 micrometer. They can include nanoparticles, emulsion droplets, foam bubbles, or other dispersed entities.
3. Composition:
• Micelles: micelles are composed of amphiphilic molecules, which have both hydrophilic and hydrophobic regions within the same molecule. The hydrophobic regions aggregate in the core of the micelle, while the hydrophilic regions form a shell around it.
• Colloidal particles: colloidal particles can have diverse compositions, including solid particles, liquid droplets, or gas bubbles. They can be composed of a single material or a combination of different components, such as core-shell nanoparticles or emulsion droplets with distinct phases.
4. Structure:
• Nicelles: micelles have a characteristic structure with a core-shell arrangement. The hydrophobic core serves as a solubilization site for hydrophobic substances, while the hydrophilic shell interacts with the surrounding medium.
• Colloidal particles: the structure of colloidal particles can vary widely depending on their composition. They can have homogeneous structures, core-shell structures, or even more complex and heterogeneous structures.
5. Behavior:
• Micelles: micelles are dynamic structures that can undergo constant rearrangements, including the exchange of molecules between micelles. They can solubilize hydrophobic substances, stabilize emulsions, and facilitate the transport of lipids and other molecules in biological systems.
• Colloidal particles: colloidal particles exhibit diverse behaviors depending on their composition. They can scatter light, display unique optical properties, exhibit Brownian motion, undergo sedimentation or flocculation, and interact with other particles or surfaces.
Understanding the differences between micelles and colloidal particles is essential for their proper utilization and application in various fields. Micelles are often associated with self-assembled structures formed by amphiphilic molecules, while colloidal particles encompass a broader range of dispersed systems with diverse compositions and origins.
Importance of understanding the difference between micelles and colloidal particles
Understanding the difference between micelles and colloidal particles is crucial for several reasons:
1. Scientific research: micelles and colloidal particles play significant roles in various scientific fields, including chemistry, materials science, and pharmaceutical research. Understanding their differences enables researchers to design experiments and develop new techniques tailored to specific systems. This knowledge contributes to advancements in nanotechnology, drug delivery systems, and formulation sciences.
2. Industrial applications: micelles and colloidal particles find extensive applications in industries such as pharmaceuticals, food and beverage, cosmetics, and paints. Differentiating between micelles and colloidal particles allows manufacturers to optimize their processes and formulations. The choice between using micelles or colloidal particles can impact drug solubility, stability, and targeted delivery to specific tissues.
3. Formulation and product development: colloidal systems, including emulsions, suspensions, and foams, are widely used in formulating products like creams, lotions, and beverages. Understanding the differences between micelles and colloidal particles helps formulators select the appropriate system and optimize the stability, texture, and appearance of the final product.
4. Drug delivery systems: micelles, formed by amphiphilic molecules, are utilized as carriers for poorly soluble drugs. Understanding the unique properties of micelles allows researchers to design drug delivery systems that improve drug solubility, enhance bioavailability, and target specific tissues. Differentiating micelles from colloidal particles helps in selecting the most suitable system for a particular drug and desired therapeutic outcome.
5. Environmental impact: colloidal particles play a crucial role in environmental processes, such as water treatment, soil remediation, and pollutant transport. Understanding their behavior and interactions with other substances aids in developing effective strategies for environmental remediation and pollution control.
6. Safety considerations: differentiating between micelles and colloidal particles is essential in assessing their potential risks and safety profiles. The behavior and stability of these systems can influence their toxicity, bioaccumulation, and environmental impact. Accurate characterization and understanding of their properties ensure proper risk assessment and regulatory compliance.
Understanding the difference between micelles and colloidal particles is fundamental to advancing scientific research, optimizing industrial processes, and developing safe and effective products and technologies. It enables researchers and formulators to make informed decisions and harness the unique properties of micelles and colloidal particles for various applications.
Conclusion
Micelles and colloidal particles are both types of dispersed systems, but they have distinct characteristics and behaviors. Micelles are formed by the self-assembly of amphiphilic molecules, with a core-shell structure and sizes typically ranging from 1 to 100 nanometers.
They solubilize hydrophobic substances and exhibit dynamic behavior. Colloidal particles encompass a broader size range (1 nm to 1 µm) and can have diverse compositions, including solid particles, liquid droplets, or gas bubbles. They can exhibit different structures and behaviors depending on their composition and origin.
