Compounds Definition of Rhombic and Monoclinic Sulphur
Rhombic Sulfur: Rhombic sulfur (also referred to as “a-sulfur”) is one of the allotropes of sulfur and its most stable allotrope at room temperatures and pressure conditions. The name derives from its characteristic crystal shape – typically diamond-shaped or rhombic in appearance).
Monoclinic Sulfur: Monoclinic sulfur (also referred to as “b-sulfur”) is another allotrope of sulfur that forms at temperatures between 96degC and 119degC, due to less stability compared with rhombic sulfur and only exists at these specific temperatures. Monoclinic sulfur’s unique crystal structure features needle-like crystals.
Rhombic and monoclinic sulfurs represent two different forms of elemental sulfur that differ primarily in terms of crystal structures and stability, making an understanding of these forms crucial for industrial applications and chemical reactions involving sulfur.
A brief overview of sulfur and its allotropes
Sulfur is a chemical element with the symbol S and atomic number 16. Sulfur, one of the Earth’s abundant nonmetals, has long been recognized by humans and plays a significant role in various industries and applications worldwide.
Sulfur exhibits several allotropes, which are different structural forms of the same element. These allotropes differ in their physical and chemical properties, making sulfur a versatile element with diverse applications.
The most common allotropes of sulfur include:
Rhombic Sulfur (α-sulfur): Rhombic sulfur is the most stable and commonly found allotrope of sulfur at room temperature. It features a crystal structure composed of sulfur atoms arranged in rhombic or diamond-shaped crystals. Rhombic sulfur is yellow in color and relatively stable.
Monoclinic Sulfur (b-Sulfur): Monoclinic sulfur has lower stability than its rhombic counterpart and exists within an optimal temperature range from 96degC to 119degC. It has a crystal structure characterized by needle-like crystals and is also yellow in color. Monoclinic sulfur undergoes a transition to rhombic sulfur when cooled below 96°C.
Plastic Sulfur (γ-sulfur): Plastic sulfur is an elastic and noncrystalline form of sulfur. It is formed when molten sulfur is rapidly cooled. Plastic sulfur can revert to the rhombic form upon heating and is capable of returning to its original shape after being deformed.
Amorphous Sulfur: Amorphous sulfur does not have a defined crystal structure and lacks long-range order. It is formed by rapidly cooling molten sulfur or by precipitating sulfur from a solution. Amorphous sulfur is often yellow or brown and is used in various chemical processes.
These different allotropes of sulfur have distinct physical and chemical properties, which contribute to their varied applications. Understanding the characteristics of each allotrope is crucial for utilizing sulfur effectively in industries such as agriculture, chemicals, pharmaceuticals, and materials science.
Importance of understanding the different forms of sulfur
Understanding the different forms of sulfur, or its allotropes, is important for several reasons:
Industrial Applications: Different forms of sulfur have specific properties that make them suitable for various industrial applications. For example, rhombic sulfur is commonly used in the production of sulfuric acid, rubber vulcanization, and the manufacturing of fertilizers. Monoclinic sulfur, on the other hand, finds applications in the production of gunpowder, matches, and certain types of explosives. Understanding these different forms allows industries to select the appropriate allotrope for their specific needs.
Chemical Reactions and Processes: Sulfur is essential in many chemical reactions and processes. It plays an integral part in these chemical transformations and reactions. The reactivity and behavior of sulfur can vary depending on its allotrope. Knowledge of the different forms of sulfur helps chemists and researchers predict and control the outcomes of these reactions. It allows for the optimization of processes and the development of new chemical compounds and materials.
Materials Science: Sulfur allotropes have unique physical and chemical properties that make them valuable in materials science research. Scientists who gain an in-depth knowledge of sulfur’s various forms can discover its many applications for energy storage, catalysis, and electronic devices. For example, plastic sulfur’s elastic and noncrystalline nature makes it suitable for certain applications in materials engineering.
Geological Relevance: Sulfur occurs naturally in many geological formations, from sulfur deposits and volcanoes to hydrothermal vents and hydrothermal pools. Its significance in these environments cannot be overemphasized. The understanding of sulfur allotropes aids in the identification and characterization of sulfur-rich minerals and ores. This knowledge is essential in mining operations and geological surveys to determine the economic viability and potential environmental impacts associated with sulfur extraction.
Environmental Effect: Sulfur compounds like sulfur dioxide (SO2) have an enormous environmental footprint and potentially severe adverse health repercussions, contributing to air pollution as well as having negative physical health implications on humans. Understanding different forms of sulfur helps us devise effective plans to lower emissions, such as extracting sulfur compounds from industrial gases or developing cleaner sulfur-based energy sources.
Comprehending the different forms of sulfur is essential for optimizing industrial processes, predicting chemical reactions, exploring new materials, and managing environmental impacts.
It enables scientists, engineers, and policymakers to make informed decisions and advancements in various fields related to sulfur utilization and sustainability.
Rhombic Sulfur
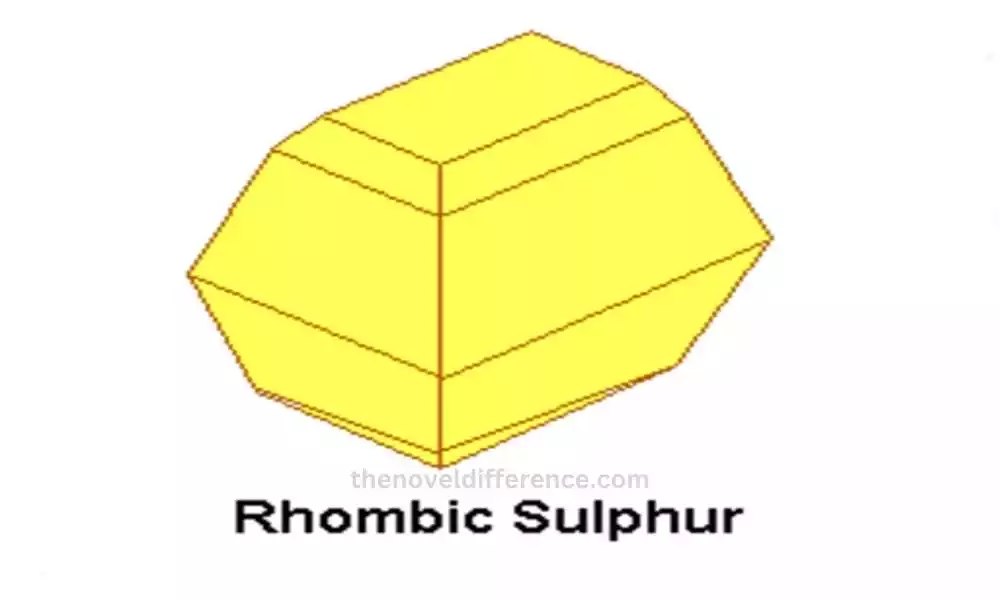
Rhombic sulfur (also referred to as a-sulfur) is one of the allotropes of sulfur. It is the most common and stable form of sulfur at room temperature and pressure. Rhombic sulfur gets its name from the shape of its crystals, which are typically rhombic or diamond-shaped.
Characteristics:
Crystal Structure: Rhombic sulfur has a crystalline structure composed of sulfur atoms arranged in a rhombic lattice. The atoms are arranged in layers with a puckered structure, giving the crystals their characteristic shape.
Color and Appearance: Rhombic sulfur is bright yellow in color. It commonly appears as powdery or crystalline yellow solids.
Properties:
Density and Melting Point: Rhombic sulfur has a density of around 2.07 grams per cubic centimeter (g/cm³). It melts at a temperature of approximately 115.21°C (239.38°F).
Stability and Reactivity: Rhombic sulfur is relatively stable and less reactive compared to other forms of sulfur. Water-insoluble, it dissolves only when mixed with organic solvents like carbon disulfide. It undergoes slow oxidation when exposed to air, forming sulfur dioxide.
Occurrence and Uses:
Natural Occurrence: Rhombic sulfur is commonly found in volcanic regions and sulfur deposits. It can also be produced through the precipitation of dissolved sulfur compounds from hot springs and hydrothermal vents.
Industrial Applications: Rhombic sulfur has various industrial applications. Sulfuric acid, which plays an indispensable role across many industries, is produced primarily using this process. Additionally, rhombic sulfur is used in the manufacturing of rubber vulcanization agents, dyes, pharmaceuticals, and agricultural fertilizers.
Rhombic sulfur’s stability, abundance, and wide range of applications make it an important allotrope of sulfur. Its properties and behavior are extensively studied and utilized in various industries, contributing to technological advancements and chemical processes.
Occurrence and uses
Occurrence: Rhombic sulfur occurs naturally in several forms and is found in various geological settings. It is commonly found in sulfur-rich volcanic regions and associated with volcanic gases and fumaroles.
Large sulfur deposits can be found in regions such as the Gulf of Mexico, Sicily, and Indonesia. Rhombic sulfur can also precipitate from hydrothermal fluids in hot springs and geothermal areas.
Uses:
Sulfuric Acid Production: One of the primary uses of rhombic sulfur is in the production of sulfuric acid. Sulfuric acid is an indispensable industrial chemical used for creating fertilizers, detergents, dyes, and explosives among various other uses. Rhombic sulfur can be burned to produce sulfur dioxide gas, which in turn converts to sulfur trioxide before being combined with water to form sulfuric acid and used in further chemical reactions that produce its derivative.
Rubber Vulcanization: Rhombic sulfur is extensively used in the vulcanization process of rubber. Vulcanization involves cross-linking rubber molecules using sulfur, leading to improved mechanical properties, durability, and resistance to heat and aging. The rhombic sulfur is mixed with the rubber compound and heated to initiate the vulcanization reaction.
Agricultural Fertilizers: Sulfur is an essential nutrient for plant development and its use is found in agricultural fertilizer production. Rhombic sulfur has proven itself as a cost-efficient means of creating superior results in terms of productivity. It is often incorporated into fertilizers to provide sulfur to crops and improve soil fertility. Sulfur deficiency in plants can result in reduced crop yield and quality.
Pharmaceuticals and Chemical Synthesis: Rhombic sulfur is used in the production of pharmaceuticals and various chemical compounds. It serves as a starting material for the synthesis of sulfur-containing organic compounds, including drugs, pesticides, and specialty chemicals.
Laboratory and Research Applications: Rhombic sulfur is commonly used in laboratory experiments and research studies. Due to its well-defined crystal structure and stable properties, quartz makes an excellent material for studying crystallography, chemical reactions, and materials science research.
Other Applications: Rhombic sulfur finds uses in other applications such as the production of pigments, dyes, and sulfur-based chemicals. It is also utilized in certain pyrotechnic formulations and matches.
Rhombic sulfur has significant industrial importance and plays a crucial role in the production of sulfuric acid, rubber vulcanization, agriculture, pharmaceuticals, and various chemical processes. It’s a natural occurrence and versatile properties make it a valuable resource in different sectors.
Monoclinic Sulfur
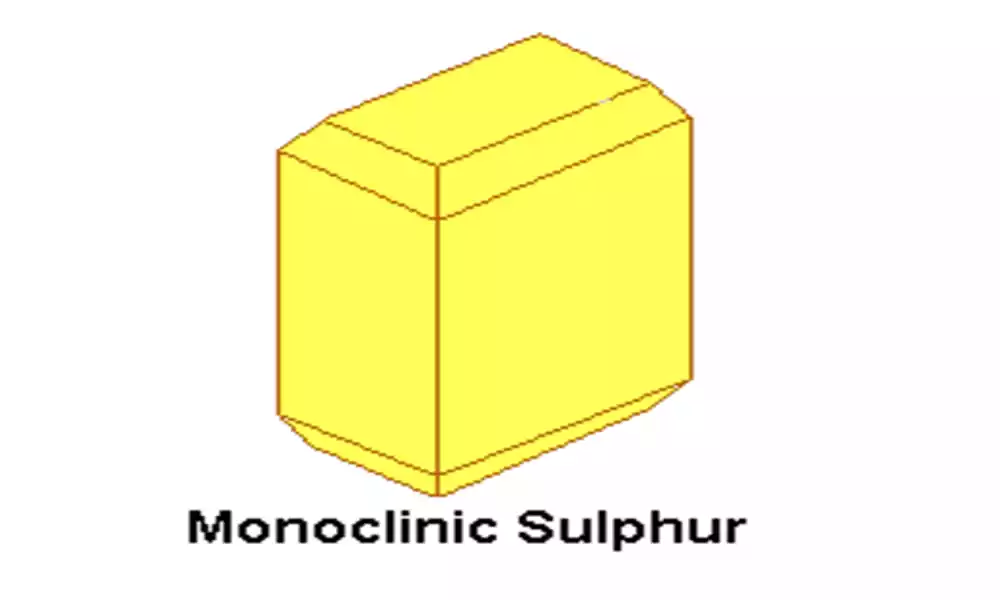
Monoclinic sulfur, also known as β-sulfur, is an allotrope of sulfur that exists in a different crystal structure compared to rhombic sulfur. It is less stable than rhombic sulfur and has distinct properties and applications.
Characteristics:
Crystal Structure: Monoclinic sulfur has a monoclinic crystal structure, characterized by needle-like crystals. The sulfur atoms are arranged differently compared to rhombic sulfur, resulting in their unique crystal shape.
Color and Appearance: Monoclinic sulfur is also yellow in color, similar to rhombic sulfur, and often appears as fine needles or elongated crystals.
Properties:
Density and Melting Point of Monoclinic Sulfur: Monoclinic sulfur has an estimated higher density than its rhombic counterpart at approximately 2.1 grams per cubic centimeter (g/cm3). It has a melting point of approximately 119°C (246°F).
Stability and Reactivity: Monoclinic sulfur is less stable than rhombic sulfur and undergoes a transition to rhombic sulfur when cooled below 96°C. It is more reactive than rhombic sulfur and exhibits increased reactivity toward other chemicals and substances.
Occurrence and Uses:
Natural Occurrence: Monoclinic sulfur can be found in certain sulfur deposits and volcanic regions, similar to rhombic sulfur. However, its occurrence is relatively less common compared to rhombic sulfur.
Gunpowder and Explosives: Monoclinic sulfur has historical significance in the production of gunpowder and explosives. It was traditionally used as an ingredient in black powder formulations due to its combustion properties and ability to support rapid chemical reactions.
Matches: Monoclinic sulfur is used in the production of certain types of matches. The sulfur component in matches aids in the ignition process by providing the necessary combustible material.
Chemical Synthesis and Research: Monoclinic sulfur is utilized in chemical synthesis and research applications. Its unique properties and reactivity make it valuable in the development of sulfur-containing compounds, catalysts, and materials science studies.
It’s important to note that while rhombic sulfur is the more prevalent and stable form at room temperature, monoclinic sulfur’s distinct crystal structure, and reactivity provide specific applications in certain industries and research fields.
Occurrence and uses
Occurrence:
Monoclinic sulfur, or β-sulfur, occurs naturally in certain sulfur-rich geological environments. It is found in sulfur deposits, volcanic regions, and hydrothermal areas. Monoclinic sulfur can precipitate from hot springs and hydrothermal fluids, especially in areas with high sulfur content.
Uses:
Gunpowder and Explosives: Monoclinic sulfur has been historically used in the production of gunpowder and explosives. It serves as a combustible ingredient that provides the necessary sulfur component for rapid chemical reactions and energy release.
Matches: Monoclinic sulfur is used in the manufacture of certain types of matches. The sulfur component plays a crucial role in the ignition process by easily catching fire and aiding the combustion of other match components.
Chemical Synthesis and Research: Monoclinic sulfur is employed in various chemical synthesis processes and research applications. Its reactivity and unique properties make it useful for the production of sulfur-containing compounds, catalysts, and materials science studies. It can be utilized as a starting material or reactant in the synthesis of organic and inorganic compounds.
Flotation Agents: Monoclinic sulfur finds application as a flotation agent in the mining industry. It is used in the froth flotation process to separate valuable minerals from their ore. Monoclinic sulfur aids in selectively separating minerals based on their surface properties.
Soil Amendment: Sulfur is an essential nutrient for plant growth, and monoclinic sulfur can be used as a soil amendment to provide sulfur to crops. It helps correct sulfur deficiencies in soils and contributes to healthy plant growth and development.
Analytical Chemistry: Monoclinic sulfur can be employed in analytical chemistry techniques. It is sometimes used as a standard or reference material for calibration purposes in sulfur analysis methods.
While the applications of monoclinic sulfur are not as extensive as those of rhombic sulfur, it still has specific uses in the production of explosives, matches, chemical synthesis, flotation processes, soil amendment, and analytical chemistry. Its occurrence in certain geological settings allows for its utilization in various industrial and scientific contexts.
Differences between Rhombic and Monoclinic Sulfur
Key differences between rhombic sulfur and monoclinic sulfur include:
Crystal Structure: Rhombic sulfur features an irregular crystal structure composed of diamond- or rhombic-shaped crystals while monoclinic sulfur has needle-shaped crystals. Rhombic sulfur has a layered arrangement of sulfur atoms, whereas monoclinic sulfur has a different arrangement of atoms in its crystal lattice.
Stability: Rhombic sulfur is more stable than monoclinic sulfur. Rhombic sulfur is the most common and stable form of sulfur at room temperature and pressure. Monoclinic sulfur, on the other hand, is less stable and undergoes a transition to rhombic sulfur when cooled below 96°C.
Melting Point: Rhombic sulfur has a lower melting point compared to monoclinic sulfur. Rhombic sulfur melts at approximately 115.21 degrees C (239.38 degrees F), while monoclinic has an approximate melting point of around 119 degrees C (246 degrees F).
Reactivity: Monoclinic sulfur is generally more reactive than rhombic sulfur. It exhibits increased reactivity towards other substances, making it more prone to chemical reactions and transformations. Rhombic sulfur is relatively less reactive and exhibits greater stability.
Appearance: Rhombic sulfur typically appears as powdery or crystalline yellow solids, while monoclinic sulfur is also yellow but often seen as fine needle-like crystals.
Occurrence: Rhombic sulfur is the more common form of sulfur found in nature, occurring in sulfur-rich volcanic regions and deposits. Monoclinic sulfur occurs less frequently and is typically associated with specific geological environments, including sulfur deposits and hydrothermal areas.
Applications: Rhombic sulfur is widely used in the production of sulfuric acid, rubber vulcanization, and various industrial processes. Monoclinic sulfur has historical applications in gunpowder and explosives, as well as matches. Rhombic sulfur has a broader range of industrial uses compared to monoclinic sulfur.
These differences in crystal structure, stability, reactivity, appearance, occurrence, and applications distinguish rhombic sulfur from monoclinic sulfur. Understanding these distinctions is important for selecting the appropriate form of sulfur for specific applications and research purposes.
What are the similarities between Rhombic and Monoclinic Sulphur?
Despite their differences, rhombic sulfur and monoclinic sulfur share several similarities:
Chemical Composition: Rhombic sulfur and monoclinic sulfur are both forms of elemental sulfur, consisting of S8 molecules. The sulfur atoms are bonded together to form stable sulfur rings.
Color: Both rhombic sulfur and monoclinic sulfur are yellow in color. Their yellow appearance is a characteristic of elemental sulfur.
Allotropes of Sulfur: Rhombic sulfur and monoclinic sulfur are two of the several allotropes of sulfur. Allotropy refers to the ability of an element to exist in different structural forms while retaining the same chemical composition.
Sulfur Transition: Monoclinic sulfur can undergo a transition to rhombic sulfur when cooled below 96°C. This transition occurs due to changes in the arrangement of sulfur atoms, allowing monoclinic sulfur to convert into a more stable rhombic form.
Industrial Applications: Both rhombic sulfur and monoclinic sulfur have industrial applications, although rhombic sulfur has a broader range of uses. Soda lime can also be found in rubber vulcanization agents, matches, and certain explosives for manufacturing processes.
Natural Occurrence: Rhombic sulfur and monoclinic sulfur occur naturally in sulfur-rich geological environments. They are found in sulfur deposits, volcanic regions, and hydrothermal areas.
While there are notable differences between rhombic sulfur and monoclinic sulfur, their shared chemical composition, yellow color, allotropic nature, industrial applications, and natural occurrence highlight the commonalities between these two forms of sulfur.
Rhombic vs Monoclinic Sulphurin Tabular Form
Sure! Here’s a tabular form comparing the key characteristics of rhombic sulfur and monoclinic sulfur:
| Characteristic | Rhombic Sulfur | Monoclinic Sulfur |
|---|---|---|
| Crystal Structure | Rhombic | Monoclinic |
| Appearance | Rhombic or diamond- | Needle-like crystals |
| shaped crystals | ||
| Color | Yellow | Yellow |
| Stability | More stable | Less stable |
| Melting Point | Around 115.21°C | Approximately 119°C |
| Reactivity | Relatively less | More reactive |
| reactive | ||
| Occurrence | Common | Less common |
| Industrial | Sulfuric acid | Gunpowder and |
| Applications | production, rubber | explosives match, |
| vulcanization, | certain chemical | |
| industrial uses | syntheses |
Please be aware that this table provides only an overall comparison, with individual values possibly deviating depending on source and conditions.
Applications and Significance
Applications and significance of rhombic and monoclinic sulfur:
Applications:
Rhombic Sulfur:
Sulfuric Acid Production: Rhombic sulfur is used extensively in the manufacturing of sulfuric acid, an essential industrial chemical utilized for various sectors including fertilizers, detergents, and chemical manufacturing.
Rubber Industry: Rhombic sulfur is crucial in the vulcanization process of rubber, improving its strength, elasticity, and resistance to heat and aging.
Fertilizer Manufacturing: Sulfur is an essential nutrient for plants, and rhombic sulfur is utilized in the production of sulfur-based fertilizers, correcting sulfur deficiencies in soil.
Pharmaceutical Industry: Rhombic sulfur serves as a starting material for the synthesis of various sulfur-containing compounds used in pharmaceutical production.
Chemical Reactions and Processes: Rhombic sulfur finds applications in chemical reactions, including the synthesis of dyes, pigments, and specialty chemicals.
Monoclinic Sulfur:
Gunpowder and Explosives: Monoclinic sulfur has long been used as an ingredient to manufacture gunpowder and various explosive products due to its superior combustion characteristics.
Matches: Monoclinic sulfur is utilized in the production of matches, providing the combustible material needed for ignition.
Chemical Synthesis: Monoclinic sulfur can be utilized in chemical synthesis processes to generate various sulfur-containing compounds and catalysts.
Flotation Agents: Monoclinic sulfur finds application as a flotation agent in the mining industry to separate valuable minerals from their ores.
Analytical Chemistry: Monoclinic sulfur is used as a reference material in sulfur analysis methods for calibration purposes.
Significance:
Industrial Importance: Both rhombic and monoclinic sulfur play critical roles in various industries, including chemical manufacturing, rubber production, agriculture, and mining.
Elemental Sulfur Source: Sulfur in its various forms is an indispensable source of elemental sulfur that’s used for making chemicals and materials with sulfur components.
Materials Science and Research: Understanding the properties and behavior of rhombic and monoclinic sulfur contributes to materials science research, catalysis studies, and the development of new compounds and materials.
Environmental Impact: Sulfur and its compounds have environmental significance, particularly in terms of emissions and pollution. Understanding the applications and properties of sulfur helps in managing and mitigating environmental impacts.
The applications and significance of rhombic and monoclinic sulfur span across industries, research fields, and environmental considerations, showcasing their importance in various domains.
Conclusion
Rhombic and Monoclinic Sulphur are two distinct allotropes of elemental sulfur, each possessing unique characteristics and applications.
Rhombic sulfur is the more stable and commonly found form at room temperature, with a rhombic crystal structure and a wide range of industrial uses such as sulfuric acid production and rubber vulcanization.
Monoclinic sulfur, on the other hand, is less stable and has a needle-like crystal structure, historically employed in gunpowder, explosives, and matches.
