When it comes to chemistry, understanding the various concepts and properties of elements is crucial. Two such concepts that often confuse are catenation and tetravalency. In this article, we will delve into the differences between catenation and tetravalency and explore their significance in the world of chemistry.
Definition of Catenation and Tetravalency
Catenation: Catenation alludes to the capacity of a component, most outstandingly carbon, to make covalent bonds with other particles of the same component, coming about within the arrangement of long chains or rings. This unique property allows for the creation of diverse organic compounds. Carbon, in particular, is known for its exceptional catenation capabilities, giving rise to a wide range of organic molecules with different structures and properties.
Tetravalency: Tetravalency alludes to the capacity of a component to create four covalent bonds with other iotas. In the context of organic chemistry, tetravalency is often associated with carbon. Carbon has four valence electrons in its outermost energy level, enabling it to share electrons with other atoms and form stable compounds by creating four covalent bonds. This characteristic makes carbon flexible because it can bond with an assortment of other components, counting hydrogen, oxygen, nitrogen, and numerous others, to create complex natural atoms. Other elements, such as silicon and germanium, also exhibit tetravalency and share similar chemical properties with carbon.
Importance of understanding the difference between catenation and tetravalency
Understanding the difference between catenation and tetravalency is important for several reasons:
1. Chemical Structure and Bonding: Catenation and tetravalency are fundamental concepts that underpin the structure and bonding in organic compounds. Catenation allows carbon and other elements to form long chains or rings, giving rise to diverse molecular structures. Tetravalency, on the other hand, determines the number of bonds an element can form and its ability to create stable compounds. Understanding these concepts helps in predicting and explaining the structures and properties of organic molecules.
2. Organic Chemistry: Catenation and tetravalency are key principles in organic chemistry. Carbon’s ability to catenate and be tetravalent is the basis for the immense complexity and diversity of organic compounds. It permits the arrangement of useful bunches, such as alkanes, alkenes, alkynes, and fragrant rings, which play vital parts in natural responses and the amalgamation of complex particles. By understanding these concepts, chemists can plan and control natural compounds for different applications, counting pharmaceuticals, materials, and vitality.
3. Biological Systems: Catenation and tetravalency are also relevant in the study of biological systems. Natural atoms, such as proteins, nucleic acids (DNA and RNA), carbohydrates, and lipids, are basic for life and display perplexing structures and capacities. Understanding catenation and tetravalency helps in deciphering the molecular architecture and interactions within biological systems, providing insights into biochemical processes, drug interactions, and disease mechanisms.
4. Material Science and Engineering: Catenation and tetravalency have implications beyond organic chemistry. Elements like silicon, with catenation and tetravalency properties, are vital in material science and engineering. Silicon is broadly utilized within the semiconductor industry for the generation of computer chips and electronic gadgets. Understanding these concepts helps with the planning and advancement of modern materials with particular properties, such as quality, conductivity, and adaptability.
5. Industrial Applications: Knowledge of catenation and tetravalency is crucial in various industrial sectors. The ability to manipulate organic compounds with specific structures and properties is essential in pharmaceuticals, where drug design and synthesis rely on understanding the relationships between molecular structure and biological activity. Additionally, polymers, plastics, and materials industries leverage the principles of catenation and tetravalency to create new materials with tailored properties for applications in packaging, construction, and consumer goods.
Understanding the difference between catenation and tetravalency is foundational to comprehending the structure, bonding, and reactivity of organic compounds. It has wide suggestions over disciplines such as chemistry, science, materials science, and mechanical applications, empowering progressions in different logical, mechanical, and commercial endeavors.
What is Catenation?
Catenation alludes to the capacity of certain components, particularly carbon, to create bonds with other iotas of the same component. This results in the formation of long chains or rings, known as catenated compounds. Carbon is the most well-known element that exhibits catenation, but other elements such as silicon, sulfur, and phosphorus also possess this property.
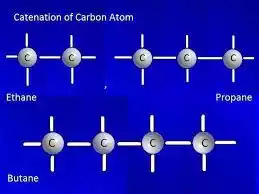
Catenation in Carbon
Carbon is known for its remarkable capacity to catenate, driving the arrangement of a wide run of natural compounds. This one-of-a-kind property of carbon is ascribed to its electronic setup, which permits it to create steady covalent bonds with other carbon molecules. As a result, carbon-based molecules can have complex structures and diverse chemical properties.
Examples of Catenated Compounds
A few cases of catenated compounds incorporate hydrocarbons like alkanes, alkenes, and alkynes, as well as naturally useful bunches like alcohols, aldehydes, and carboxylic acids. These compounds play an essential part in different chemical forms and are the building squares of life.
Carbon is a primary example of catenation
Carbon is indeed the primary example of catenation. It is known for its remarkable ability to form long chains, branched structures, and rings by bonding with other carbon atoms. This property of carbon plays a pivotal part in natural chemistry and is capable of the endless differing qualities of natural compounds found in nature and made artificially.
Carbon has four valence electrons in its outermost energy level, allowing it to form up to four covalent bonds. These bonds can be single, twofold, or triple bonds, depending on the number of shared electrons. When carbon atoms bond with each other, they can form stable chains or cyclic structures.
Carbon chains can be linear or branched, and their lengths can vary from a few carbon atoms to thousands. For example, in alkanes, which are saturated hydrocarbons, carbon atoms are linked in a straight or branched chain. Methane (CH4) is the best alkane, with a single carbon iota, whereas ethane (C2H6) and propane (C3H8) have two and three carbon particles, separately. The carbon atoms in these compounds are connected by single bonds.
Carbon can also form cyclic structures or rings. In cyclic compounds, carbon atoms are bonded in a ring formation, creating structures like cycloalkanes and aromatic compounds. Benzene (C6H6) is an illustration of a fragrant compound, comprising a hexagonal ring of carbon iotas with rotating single and twofold bonds.
The ability of carbon to catenate and form these diverse structures is the foundation of organic chemistry. It permits the creation of complex natural atoms found in living beings, counting proteins, nucleic acids, carbohydrates, and lipids. Carbon’s catenation property empowers the amalgamation of a wide extend of natural compounds, such as pharmaceuticals, polymers, colors, and solvents, with different properties and applications.
Carbon’s exceptional catenation ability, coupled with its tetravalency, makes it a key element in organic chemistry, providing the basis for the complexity and versatility of organic compounds.
Other elements exhibiting catenation
While carbon is the most prominent element known for its catenation ability, several other elements also exhibit catenation to varying degrees.
These elements include:
1. Silicon (Si): Silicon is often referred to as the “second carbon” due to its similar catenation properties. It has four valence electrons like carbon and can form long chains and cyclic structures by bonding with other silicon atoms. Silicones, which are silicon-based polymers, are an example of compounds formed through silicon catenation.
2. Sulfur (S): Sulfur is another element that can exhibit catenation. While it is not as extensive as carbon or silicon, sulfur can form chains and rings by bonding with other sulfur atoms. Examples include cyclic sulfur compounds like cyclooctasulfur (S8) and long chains of sulfur atoms found in polysulfide compounds.
3. Phosphorus (P): Phosphorus can also display catenation properties to some extent. It can form chains and rings by bonding with other phosphorus atoms, giving rise to compounds such as cyclic phosphorus compounds and polyphosphates.
It is important to note that while these elements can exhibit catenation, carbon remains the most prominent and versatile element in terms of its ability to form extensive chains, rings, and diverse organic compounds. The catenation observed in other elements may not be as extensive or versatile as carbon’s, but it still contributes to the variety of compounds and structures found in chemistry.
What is Tetravalency?
Tetravalency alludes to the capacity of certain components, such as carbon and silicon, to make four covalent bonds with other particles. The term “valency” refers to the number of bonds an atom can form. Tetravalent elements have four valence electrons available for bonding, allowing them to achieve a stable electronic configuration by sharing electrons with other atoms.
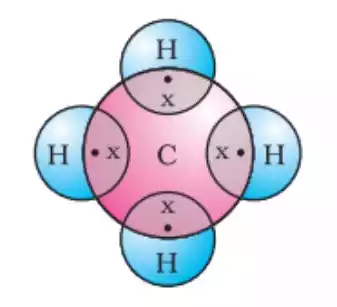
Tetravalency in Carbon
Carbon is the most well-known tetravalent element. With four valence electrons, carbon can shape steady bonds with other carbon particles, as well as with other components like hydrogen, oxygen, nitrogen, and incandescent light. This versatility makes carbon the backbone of organic chemistry and the foundation for the incredible diversity of organic compounds found in nature.
Tetravalent Compounds
Tetravalent compounds are widely found in nature and have numerous applications. Silicon, another tetravalent component, is broadly utilized within the semiconductor industry due to its capacity to create steady covalent bonds with other silicon molecules.
Explanation of tetravalent elements
Tetravalent elements are those that can form four covalent bonds with other atoms. This property allows these elements to achieve a stable electron configuration by completing their valence shells with eight electrons, following the octet rule.
Carbon (C) is the most well-known tetravalent element. It has four valence electrons in its outermost energy level (2s^2 2p^2), which allows it to form four covalent bonds. By sharing these valence electrons with other atoms, carbon can fill its outermost energy level with eight electrons, achieving a stable configuration. Carbon’s ability to form multiple bonds, such as single, double, and triple bonds, further enhances its versatility in creating diverse compounds.
Silicon (Si) is another important tetravalent element. It has four valence electrons (3s^2 3p^2) and shares similar properties to carbon. Silicon can form four covalent bonds by sharing its valence electrons, resulting in stable compounds. Silicon’s tetravalency makes it a key element in the semiconductor industry, as it can create materials with unique electronic properties.
Other examples of tetravalent elements include germanium (Ge), tin (Sn), and lead (Pb). These elements, like carbon and silicon, have four valence electrons in their outermost energy levels. They can form four covalent bonds by sharing these valence electrons, allowing them to create stable compounds with diverse structures and properties.
Tetravalent elements play significant roles in various fields. They are fundamental for the arrangement of complex natural particles and utilitarian bunches. These elements also have applications in materials science, electronics, and the production of semiconductor devices.
It is imperative to note that whereas carbon is the foremost conspicuous and broadly considered tetravalent component, other tetravalent components display comparable chemical behavior and properties. The tetravalency of these elements contributes to their ability to form stable compounds and participate in various chemical reactions.
Significance of tetravalency in organic chemistry
Tetravalency is of immense significance in organic chemistry. It is a key property of carbon and other tetravalent elements that allows the formation of diverse organic compounds.
Here are some reasons why tetravalency is crucial in organic chemistry:
1. Formation of Covalent Bonds: Tetravalent elements, particularly carbon, can form up to four covalent bonds by sharing their valence electrons. This enables the creation of stable molecular structures in organic compounds. Covalent bonds are strong and directional, providing stability to organic molecules and allowing them to participate in various chemical reactions.
2. Versatile Molecular Architectures: Tetravalency gives carbon the ability to form long chains, branched structures, and rings. This versatility in molecular architecture is the basis for the vast diversity of organic compounds. Carbon chains can vary in length, allowing for compounds of different sizes and properties. The formation of rings and cyclic structures further expands the range of possible structures in organic chemistry.
3. Functional Group Diversity: Tetravalency allows carbon to bond with different functional groups, which are specific arrangements of atoms within a molecule that confer unique chemical properties and reactivity. Functional groups play a crucial role in organic reactions and determine the behavior and properties of organic compounds. Tetravalency permits the connection of utilitarian bunches to carbon, driving to the tremendous cluster of utilitarian bunches found in natural chemistry, such as alcohols, aldehydes, ketones, carboxylic acids, and amines.
4. Stability of Organic Compounds: Tetravalent elements contribute to the stability of organic compounds. By forming four covalent bonds, carbon achieves a stable electron configuration with a complete octet. This stability makes organic compounds less reactive compared to many inorganic compounds, which can have higher reactivity due to incomplete valence shells.
5. Synthesis of Complex Molecules: Tetravalency allows for the synthesis of complex organic molecules found in living organisms and created synthetically. Natural compounds such as proteins, nucleic acids (DNA and RNA), carbohydrates, and lipids are fundamental components of life and show perplexing structures and capacities. The ability to form multiple bonds and diverse structures through tetravalency enables the synthesis of these complex molecules, leading to a better understanding of biological systems and the development of pharmaceuticals, agrochemicals, and biomaterials.
Tetravalency is fundamental to organic chemistry as it enables the formation of stable covalent bonds, diverse molecular architectures, and functional groups. This property of carbon and other tetravalent elements is instrumental in the synthesis of complex organic compounds, contributing to advancements in medicine, materials science, and various other fields.
Comparison Chart
Sure! Here’s a comparison chart highlighting the key differences between catenation and tetravalency:
| Aspect | Catenation | Tetravalency |
|---|---|---|
| Definition | The ability of an element to form long chains or rings with itself | Property of an element to form four covalent bonds with other atoms |
| Elements | Carbon, Silicon, Sulfur (to some extent) | Carbon, Silicon, Germanium, Tin, Lead |
| Nature of Bonds | Covalent bonds within the same element | Covalent bonds between different elements |
| Molecular Structures | Long chains, branched structures, rings | Varied molecular structures |
| Significance | Enables the formation of diverse organic compounds and complexity in molecular structures | Determines the number of bonds an element can form and its stability in compounds |
| Scope | Primarily observed in carbon and a few other elements | Applicable to elements across the periodic table |
This chart provides a concise overview of the main differences between catenation and tetravalency in terms of their definitions, elements involved, nature of bonds, molecular structures formed, significance, and scope of application.
Conclusion
Catenation and tetravalency are two distinct concepts in chemistry. Catenation alludes to the capacity of certain components, like carbon, to make chains or rings with iotas of the same component, whereas tetravalency refers to the capacity to make four covalent bonds. These properties play a critical part in the arrangement and differences of natural compounds, making them fundamental concepts to get within the world of chemistry.