Definition of Solidus and Liquidus
Sure! Here is an explanation of Solidus and Liquidus:
- Solidus: Solidus is a term commonly used in materials science and thermodynamics to refer to the temperature threshold at which material becomes fully solid. It represents the highest temperature at which a substance remains fully solid. At temperatures above the Solidus, the material starts to undergo partial melting and exists as a combination of solid and liquid phases.
- Liquidus: Liquidus is also a term used in materials science and thermodynamics and represents the temperature boundary or threshold above which a material is completely liquid. It signifies the lowest temperature at which a substance exists entirely in a liquid state. At temperatures below the Liquidus, the material remains solid or partially solid due to incomplete melting.
The Solidus defines the upper-temperature limit below which a material is fully solid, while the Liquidus defines the lower temperature limit above which a material is fully liquid. These concepts are crucial for understanding phase transitions, material behavior, and microstructural changes that occur during heating and cooling processes.
Importance of understanding the difference between Solidus and Liquidus
Understanding the difference between Solidus and Liquidus is crucial in materials science and various industries where the behavior and properties of materials are of utmost importance.
Here are some reasons why understanding this difference is significant:
- Phase Transitions: The Solidus and Liquidus temperatures determine the boundaries within which phase transitions occur in materials. By understanding these temperatures, researchers and engineers can predict and control the transformation of materials from solid to liquid or vice versa. This knowledge is particularly important in processes such as casting, welding, and solidification, where precise control of phase transitions is necessary to achieve desired material properties and structures.
- Material Behavior: The difference between Solidus and Liquidus has a direct impact on the behavior of materials. The range between the Solidus and Liquidus temperatures represents the temperature window in which both solid and liquid phases coexist. Understanding this range helps in comprehending phenomena like solidification, melting, and solid-state transformations, which ultimately influence the mechanical, thermal, and electrical properties of materials.
- Microstructural Evolution: The Solidus and Liquidus temperatures play a crucial role in the microstructural evolution of materials. The phase transitions occurring within the Solidus-Liquidus range affect the arrangement of atoms, crystal growth, and formation of defects or grain boundaries. By understanding the Solidus and Liquidus, researchers can manipulate and optimize microstructures to enhance material performance and properties.
- Material Processing and Design: The knowledge of Solidus and Liquidus is essential for various material processing and design techniques. For example, in alloy manufacturing, understanding the Solidus and Liquidus temperatures helps in determining the appropriate temperature range for alloy casting or heat treatment. It allows for selecting compositions that provide desirable melting and solidification behaviors, leading to improved material properties.
- Phase Diagram Analysis: Solidus and Liquidus are key points on a phase diagram, which is a graphical representation of a material’s phases and phase transitions as a function of temperature and composition. Analyzing the phase diagram enables the prediction of phase stability, identification of phase boundaries, and understanding of the composition-dependent behavior of materials. The Solidus and Liquidus temperatures serve as reference points for interpreting and utilizing phase diagrams effectively.
Understanding the difference between Solidus and Liquidus is essential for controlling phase transitions, predicting material behavior, optimizing microstructures, and designing and processing materials for specific applications.
This knowledge forms the foundation for advancements in materials science, engineering, and various industries where precise control of material properties is vital.
Solidus
The Solidus is a term used in materials science and thermodynamics to refer to the temperature boundary or threshold below which a material is completely solid. It represents the highest temperature at which a substance remains in a fully solid state.
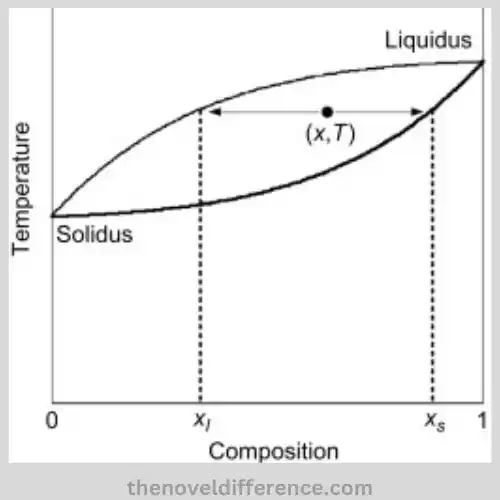
The Solidus temperature is a critical parameter for understanding the behavior and properties of materials during heating or cooling processes. It plays a significant role in phase transitions, microstructural changes, and material transformations. Below the Solidus temperature, materials exist as solid phases, and their properties are predominantly governed by the behavior of solid-state materials.
The determination of the Solidus temperature is essential for various applications and industries. It helps in understanding and controlling processes such as solidification, casting, and heat treatment.
By identifying the Solidus temperature, researchers and engineers can predict the onset of melting or phase transitions, allowing for the optimization of material processing conditions and the achievement of desired material properties.
The Solidus temperature is typically determined through experimental techniques such as differential scanning calorimetry (DSC), thermal analysis, or by analyzing phase diagrams specific to the material system of interest.
It is important to note that the Solidus temperature can vary depending on the composition of the material, as different compositions may have different melting points.
In summary, the Solidus represents the upper-temperature limit below which a material is fully solid. Understanding the Solidus temperature is crucial for predicting phase transitions, optimizing material processing, and tailoring material properties to meet specific requirements.
Liquidus
The Liquidus is a term used in materials science and thermodynamics to refer to the temperature boundary or threshold above which a material is completely liquid. It signifies the lowest temperature at which a substance exists entirely in a liquid state.
The Liquidus temperature is a critical parameter for understanding the behavior and properties of materials during heating or cooling processes. It plays a significant role in phase transitions, microstructural changes, and material transformations.
Above the Liquidus temperature, materials exist as liquid phases, and their properties are predominantly governed by the behavior of liquid-state materials.
The determination of the Liquidus temperature is essential for various applications and industries. It helps in understanding and controlling processes such as melting, casting, and crystallization.
By identifying the Liquidus temperature, researchers and engineers can predict the onset of complete liquefaction or phase transitions, allowing for the optimization of material processing conditions and the achievement of desired material properties.
The Liquidus temperature is typically determined through experimental techniques such as differential scanning calorimetry (DSC), thermal analysis, or by analyzing phase diagrams specific to the material system of interest.
Note that Liquidus temperatures vary based on material composition as different compositions have different melting points.
The Liquidus represents the lower temperature limit above which the material is fully liquid. Understanding the Liquidus temperature is crucial for predicting phase transitions, optimizing material processing, and tailoring material properties to meet specific requirements.
Difference between Solidus and Liquidus
The difference between Solidus and Liquidus lies in their respective temperature ranges and the states of matter associated with those temperatures:
- Temperature Range:
-
- Solidus: The Solidus temperature represents the upper-temperature boundary below which a material is completely solid. It is the highest temperature at which a substance remains in a fully solid state.
- Liquidus: The Liquidus temperature represents the lower temperature boundary above which the material is completely liquid. It is the lowest temperature at which a substance exists entirely in a liquid state.
- State of Matter:
-
- Solidus: Below the Solidus temperature, a material exists in a solid or partially solid state. The material may undergo phase transitions or exhibit specific solid-state properties.
- Liquidus: Above the Liquidus temperature, a material is entirely in a liquid state. The material has lost its solid structure and exhibits properties associated with liquids.
- Phase Transitions:
-
- Solidus: The Solidus temperature is the threshold at which a material starts to undergo partial melting or phase transitions from solid to liquid. It marks the beginning of the melting range.
- Liquidus: The Liquidus temperature is the threshold at which a material is fully liquid and represents the melting point of the material.
- Coexistence Range:
-
- Solidus: The temperature range between the Solidus and Liquidus is referred to as the Solidus-Liquidus range. Within this range, both solid and liquid phases coexist, and the material undergoes partial melting or solidification, depending on the heating or cooling process.
- Microstructural Changes:
-
- Solidus: Below the Solidus temperature, the material may undergo microstructural changes such as phase transformations, crystal growth, or the formation of defects or grain boundaries within the solid state.
- Liquidus: Above the Liquidus temperature, the material loses its solid structure, and its microstructure is characterized by the mobility of atoms or molecules associated with the liquid state.
The Solidus and Liquidus temperatures represent the boundaries between a material’s solid and liquid states. The Solidus temperature marks the upper-temperature limit below which a material is fully solid, while the Liquidus temperature represents the lower temperature limit above which a material is entirely liquid.
Understanding the difference between Solidus and Liquidus is crucial for controlling phase transitions, predicting material behavior, and optimizing processing conditions in various industries.
What are the Similarities Between Solidus and Liquidus?
While Solidus and Liquidus represent distinct concepts in materials science, there are certain similarities between them.
Here are some of the similarities between Solidus and Liquidus:
- Temperature Range: Both Solidus and Liquidus are temperature ranges within which materials undergo specific phase transitions. These temperature ranges are essential for understanding the behavior and properties of materials.
- Phase Diagram: Both Solidus and Liquidus are depicted on a phase diagram, which is a graphical representation of the phases and phase transitions of a material as a function of temperature and composition. The phase diagram illustrates the relationship between the Solidus and Liquidus temperatures.
- Composition Dependent: Both Solidus and Liquidus temperatures are influenced by the composition of the material. Changes in the composition can alter the Solidus and Liquidus temperatures, affecting the phases and phase transitions that occur within the material.
- Melting Point: Liquidus temperature represents the melting point of the material, or more specifically when its solid phase begins changing to the liquid phase. The Solidus temperature, on the other hand, represents the temperature below which a material exists in a solid phase.
- Boundary Interaction: The Solidus and Liquidus temperatures define the boundaries within which phase transitions occur. At the Solidus temperature, the material starts to melt, and at the Liquidus temperature, it becomes completely liquid. The temperature range between the Solidus and Liquidus is crucial for understanding the coexistence of solid and liquid phases.
- Material Transformation: Both Solidus and Liquidus are significant in determining the behavior and transformation of materials during heating or cooling processes. Understanding the Solidus and Liquidus temperatures help in predicting and controlling the phase changes, microstructural evolution, and material properties.
It’s important to note that while there are similarities between Solidus and Liquidus, they represent distinct concepts and have different implications for materials’ behavior and properties.
Conclusion
Understanding the difference between Solidus and Liquidus is of great importance in materials science. Solidus temperature measures the upper-temperature limit below which materials become fully solid; Liquidus temperature describes its lower limit above which materials become liquid-like.
These temperature boundaries define the range within which phase transitions occur and have a significant impact on the behavior, properties, and microstructural evolution of materials.
Knowledge of the Solidus and Liquidus temperatures allows researchers and engineers to predict and control phase transitions, optimize material processing conditions, and tailor material properties to meet specific requirements. It is crucial in various applications and industries such as casting, welding, solidification, and alloy manufacturing.
The Solidus and Liquidus temperatures are key reference points on phase diagrams, aiding in the interpretation of phase stability, composition-dependent behavior, and the coexistence of solid and liquid phases.
