When it comes to the world of particles and particles, various intriguing substances exist. Deuteron and Triton are two such particles that play crucial roles in the realm of nuclear physics. While they may sound similar, there are significant differences between Deuteron and Triton. We are going investigate these contrasts and shed light on the special characteristics of each molecule.
Importance of understanding the differences between the two particles
Understanding the differences between the deuteron and triton is of significant importance for several reasons:
1. Nuclear Physics: Deuteron and triton are both nucleons, which are particles found in the nucleus of an atom. By studying their properties and behavior, scientists can gain insights into the fundamental forces and interactions that govern nuclear physics. Understanding the differences between these particles contributes to our knowledge of nuclear structure and dynamics.
2. Nuclear Reactions: Deuteron and triton play crucial roles in various nuclear reactions. Deuteron is often used as a probe in scattering experiments to study the structure of atomic nuclei. Triton, on the other hand, is included in combination responses, especially within the setting of atomic vitality generation and the improvement of combination reactors. Knowing the differences between these particles allows for better modeling and understanding of such reactions.
3. Nuclear Energy: Tritium, a radioactive isotope of hydrogen that contains a triton, is a key component in fusion reactions. Fusion has the potential to provide a clean and virtually limitless source of energy. Understanding the properties and behavior of triton is essential for optimizing fusion reactions and developing efficient fusion power plants.
4. Astrophysics: Deuteron and Triton are also relevant in astrophysical contexts. In stellar nucleosynthesis, deuteron is included within the combination forms that happen in stars, driving the generation of heavier components. Triton, similarly, plays a role in certain nuclear reactions occurring in stellar environments. Understanding these processes contributes to our understanding of stellar evolution and the synthesis of elements in the universe.
5. Fundamental Particle Physics: Investigating the properties of deuteron and triton can also provide insights into the underlying structure of matter and the fundamental particles that make up the nucleus. By studying their spins, magnetic moments, and other characteristics, scientists can gain valuable information about the internal structure and properties of nucleons.
Understanding the contrasts between the deuteron and triton is pivotal for progressing our information in different logical disciplines, counting atomic material science, atomic vitality, astronomy, and principal molecule material science. This understanding allows for more accurate modeling, better experimental design, and potential applications in energy production, fusion research, and astrophysical studies.
Definition of Deuteron and Triton
Deuteron: The deuteron is a particle that is commonly referred to as a heavy hydrogen nucleus. It is the core of deuterium, an isotope of hydrogen. The deuteron consists of one proton and one neutron, bound together by a strong nuclear force. It is represented by the symbol D or ²H, where the ² indicates the mass number of 2.
Triton: The triton is a particle that is also known as a hydrogen-3 nucleus. It is an isotope of hydrogen and is represented by the symbol T or ³H, where the ³ indicates the mass number of 3. The triton comprises one proton and two neutrons, shaping a add up to three nucleons. It is a rare isotope of hydrogen and is considered a radioactive particle due to its unstable nature.
What is a Deuteron?
A deuteron is a nuclear core that comprises one proton and one neutron, bound together by the solid atomic constraint. It is commonly represented as “D” or “2H.” Deuterons are abundant in heavy water, which is composed of hydrogen isotopes, including deuterium.
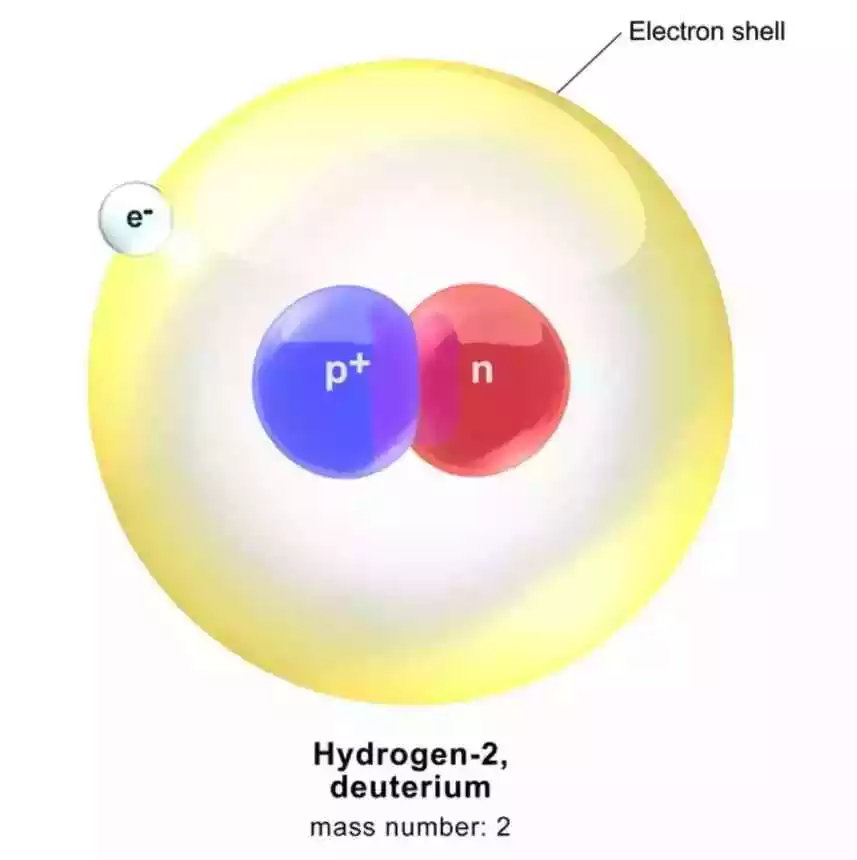
Characteristics of Deuterons
• Mass: Deuterons have a mass of approximately 2.014 atomic mass units (AMU).
• Charge: Being a combination of a proton and a neutron, deuterons carry a positive charge of +1.
• Stability: Deuterons are relatively stable and can be found in various nuclear reactions and processes.
• Usage: Deuterons are widely used in nuclear physics research, fusion reactions, and neutron production.
Nuclear properties
The deuteron possesses several important nuclear properties:
1. Charge: The deuteron carries a positive electric charge of +1e, where “e” represents the elementary charge. This charge arises from the single proton present in its nucleus.
2. Mass: The deuteron has a mass of approximately 2.014 atomic mass units (u) or 3.34 x 10^-27 kilograms. This mass arises from the combined mass of one proton and one neutron.
3. Spin: The deuteron has a spin of 1, indicating its intrinsic angular momentum. This spin arises from the combination of the spins of the proton and neutron within the nucleus.
4. Magnetic Moment: The deuteron possesses a magnetic moment resulting from the spins and orbital motions of the proton and neutron within its nucleus. The magnetic moment is a vector quantity that characterizes the strength and direction of the particle’s interaction with magnetic fields.
It is worth noticing that the deuteron may be a steady molecule, meaning it does not experience radioactive rot. Its stability allows for its use in various experimental and practical applications, such as nuclear physics research, atomic physics experiments, and studies related to nuclear fusion.
Formation and stability
Formation: Deuterons can be formed through several processes, primarily in the context of nuclear reactions. The most common method of deuteron formation is through nuclear fusion reactions involving hydrogen isotopes. When a proton and a neutron come near nearness, they can experience a solid atomic drive interaction, coming about within the arrangement of a deuteron. This fusion reaction is essential in the synthesis of deuterium, which occurs naturally in small amounts and can also be produced artificially.
Stability: Deuterons are considered stable particles since they do not undergo radioactive decay. This stability arises from the balance between the attractive strong nuclear force that binds the proton and neutron together and the repulsive electromagnetic force between the positively charged proton and the neutron. The strong nuclear force is responsible for holding the nucleons within the nucleus together, while the electromagnetic force tries to push the positively charged particles apart.
The solidness of the deuteron is assist backed by the reality that the official vitality between the proton and neutron within the deuteron is adequate to overcome any unconstrained crumbling. The official vitality of the deuteron is lower compared to bigger, more steady cores, but it is still solid sufficient to keep the deuteron intaglio.
It’s vital to note that whereas the deuteron is steady, it can still take part in atomic responses and may be influenced by outside impacts such as high-energy collisions or solid electromagnetic areas. Such as those found in high-energy particle accelerators or in stellar environments, deuteron stability can be influenced by the surrounding conditions and may undergo nuclear reactions or disintegration processes.
What is Triton?
A triton could be a molecule that speaks to the core of tritium, an isotope of hydrogen. It is also referred to as a hydrogen-3 nucleus. The triton comprises one proton and two neutrons, making it a core composed of three nucleons.
The triton is represented by the symbol T or ³H, where the ³ indicates the mass number of 3. It is considered a radioactive isotope of hydrogen due to its unsteady nature. Tritium, the component that contains a triton, rots over time through the method of beta rot, in which a neutron inside the triton changes into a proton, discharging a beta molecule (an electron) and an electron antineutrino.
Tritium is moderately uncommon, but it can be created misleadingly through different strategies, counting atomic responses including lithium or boron. Tritium contains a half-life of around 12.32 long time, meaning that over time, half of the tritium shown in a test will experience radioactive rot.
Tritons play significant roles in nuclear physics, nuclear reactions, and energy production. In certain combination responses, tritons can connect with other particles, driving the discharge of vitality and the era of more steady cores. Tritium is additionally utilized as a fuel in test and potential future combination reactors because it has the potential to supply a clean and inexhaustible source of vitality.
Nuclear properties
The triton possesses several important nuclear properties:
1. Charge: The triton carries a positive electric charge of +1e, where “e” represents the elementary charge. This charge arises from the single proton present in its nucleus.
2. Mass: The triton has a mass of approximately 3.016 atomic mass units (u) or 5.01 x 10^-27 kilograms. This mass arises from the combination of one proton and two neutrons in its nucleus.
3. Spin: The triton has a spin of 1/2, indicating its intrinsic angular momentum. This spin arises from the combination of the spins of the proton and the two neutrons within the nucleus.
4. Magnetic Moment: The triton possesses a magnetic moment resulting from the spins and orbital motions of the particles within its nucleus. The magnetic moment is a vector quantity that characterizes the strength and direction of the particle’s interaction with magnetic fields.
It is critical to note that the triton may be a radioactive molecule due to its unsteady nature. It experiences beta rot, where one of the neutrons inside the core changes into a proton, transmitting a beta molecule (an electron) and an electron antineutrino. This decay process occurs with a half-life of approximately 12.32 years.
The properties of the triton, counting its charge, mass, turn, and attractive minute, are pivotal for understanding its behavior in atomic responses, as well as its part in vitality generation through combination responses. Tritium, the component that contains tritons, is of specific intrigue in inquiries related to atomic combinations and the advancement of combination reactors.
Formation and stability
Formation: Tritons can be formed through various processes involving nuclear reactions. One common strategy of triton arrangement is through the combination of deuterium, which may be a deuteron (a core of deuterium) combined with another proton. This fusion reaction results in the creation of a triton. Tritons can also be formed through other nuclear reactions involving isotopes of hydrogen and other elements.
Stability: Whereas the triton is considered a radioactive molecule due to its unsteady nature, it features a generally long half-life compared to numerous other radioactive isotopes. The triton undergoes beta rot, where one of the neutrons within the core changes into a proton, transmitting a beta molecule (an electron) and an electron antineutrino. This decay process occurs with a half-life of approximately 12.32 years.
The solidness of the triton depends on the adjustment between the solid atomic constrain, which holds the protons and neutrons together, and the awful electromagnetic constrain between the emphatically charged protons. In the triton, the two neutrons help to stabilize the nucleus by increasing the overall attractive force between nucleons.
It’s important to note that the triton’s stability is influenced by external factors such as high-energy collisions or strong electromagnetic fields. Under certain extreme conditions, the triton may undergo nuclear reactions or disintegration processes. However, under normal conditions, the triton’s stability allows it to participate in various nuclear reactions and play important roles in nuclear physics, energy production, and other scientific studies.
Comparison Chart
Sure! Here’s a comparison chart highlighting the differences between the deuteron and triton:
| Properties | Deuteron | Triton |
|---|---|---|
| Composition | 1 proton, 1 neutron | 1 proton, 2 neutrons |
| Mass | Approximately 2.014 u | Approximately 3.016 u |
| Charge | +1e | +1e |
| Stability | Stable | Radioactive (beta decay) |
| Spin | 1 | 1/2 |
| Magnetic Moment | Present | Present |
| Formation | Fusion reactions | Fusion reactions, nuclear reactions |
Similar between Deuteron and Triton
Certainly! Here are some similarities between the deuteron and triton:
1. Nucleon Nature: Both the deuteron and triton are nucleons, meaning they are particles found in the nucleus of an atom.
2. Hydrogen Isotopes: Both particles are associated with hydrogen isotopes. The deuteron is the core of deuterium, which is the isotope of hydrogen with one proton and one neutron. The triton is the core of tritium, which is the isotope of hydrogen with one proton and two neutrons.
3. Positive Charge: Both the deuteron and triton carry a positive electric charge of +1e, where “e” represents the elementary charge.
4. Nuclear Stability: While the triton is radioactive and undergoes beta decay, the deuteron is a stable particle that does not undergo radioactive decay. Both particles can exist under normal conditions without spontaneously disintegrating.
5. Nuclear Physics Applications: Both the deuteron and triton play important roles in nuclear physics, nuclear reactions, and energy production. They are involved in various fusion reactions, scattering experiments, and studies related to nuclear structure and dynamics.
6. Magnetic Moment: Both particles possess a magnetic moment resulting from the spins and orbital motions of the protons and neutrons within their nuclei. The magnetic moments of the deuteron and triton contribute to their interactions with magnetic fields.
While there are differences between the deuteron and triton, these similarities highlight their shared characteristics and the role they play in various scientific disciplines.
Conclusion
Deuteron and Triton are two distinct particles that differ in composition, mass, stability, and applications. Deuterons, composed of one proton and one neutron, are stable and find usage in nuclear physics research, fusion reactions, and neutron production. Tritons, comprising one proton and two neutrons, are heavier and radioactive, being utilized in restorative imaging and atomic tests. Understanding the unique properties and differences between these particles enhances our knowledge of atomic nuclei and their behavior.
