Welcome to this comprehensive article where we will explore the fascinating world of endothermic and exothermic reactions. These terms might sound complex at first, but fear not! By the end of this article, you will have a clear understanding of what endothermic and exothermic reactions are and how they play a crucial role in various scientific processes. So, let’s dive right in!
Definition of Endothermic and Exothermic Reactions
Endothermic Reactions: Endothermic reactions are chemical reactions that absorb heat energy from their surroundings, resulting in a decrease in the temperature of the surrounding environment. These reactions store energy within the system, often in the form of chemical bonds.
One common example of an endothermic reaction is the process of photosynthesis, where plants absorb energy from sunlight to convert carbon dioxide and water into glucose and oxygen. This vital process sustains life on Earth by producing oxygen and providing energy-rich compounds for organisms.
Another example of an endothermic reaction is the dissolution of ammonium nitrate in water. This process absorbs heat from the surroundings, causing a significant decrease in temperature.
Exothermic Reactions: Exothermic reactions release heat energy into their surroundings, resulting in an increase in the temperature of the surrounding environment. These reactions involve the conversion of chemical potential energy into thermal energy.
Combustion reactions, such as the burning of wood or the combustion of gasoline in an engine, are prime examples of exothermic reactions. These reactions release a significant amount of heat, which is why they are used to generate energy for various applications.
Additionally, the reaction between an acid and a base, known as neutralization, is also exothermic. When an acid and a base react, they release heat energy, often observed as an increase in temperature.
Importance of understanding the difference between the Endothermic and Exothermic Reactions
Knowledge of endothermic and exothermic reactions is of great significance in several situations, Understanding this distinction will aid you greatly.
For instance:
- Anticipating and Controlling Reactions: Understanding whether a reaction is endothermic or exothermic allows scientists and chemists to predict its direction and feasibility as well as assess the energy needs necessary for its occurrence. It allows scientists and chemists to manage reactions more precisely while helping predict outcomes more reliably.
- Energy Transfer and Conservation: Both endothermic and exothermic reactions involve energy transfers between molecules in chemical reactions, giving us insight into how it moves between reactions. Our knowledge about this can be put towards conserving energy consumption by designing efficient processes or developing sustainable technologies.
- Industrial Applications: Many industrial processes rely on endothermic or exothermic reactions for production processes and cost-cutting measures, for instance, the Haber-Bosch process for ammonia production is an exothermic reaction while producing methanol involves an endothermic reactions synthesis process is an endothermic reaction, Understanding these reactions helps Optimize production processes while increasing efficiency while cutting costs.
- Environmental Implications: Endothermic and exothermic reactions have environmental ramifications; for instance, fossil fuel combustion releases heat energy which contributes to global warming and climate change. Understanding these reactions helps in developing cleaner energy solutions while mitigating their negative environmental impact.
- Application in Everyday Life: Endothermic and exothermic reactions don’t solely exist within labs and industries – they occur naturally throughout our daily lives as well. An understanding of these reactions helps explain phenomena like cooking, refrigeration, condensation, and various biological processes like respiration and photosynthesis.
- Safety Considerations: Knowing whether a reaction is endothermic or exothermic is critical for safety purposes, particularly since exothermic reactions produce large quantities of heat and energy release that must be carefully handled to avoid accidents or explosions. Understanding this distinction aids in developing suitable safety protocols and measures.
Understanding endothermic and exothermic reactions is central to successfully anticipating controlling and optimizing reactions. understanding energy transfer and conservation processes. optimizing industrial processes. addressing environmental concerns. Explaining everyday phenomena. Assuring safety when handling reactive systems. Understanding their differences provides the basis for many scientific applications across disciplines.
Endothermic Reactions
Endothermic Reactions or Chemical Processes which Absorb or Release Energy in the form of heat from their Surroundings And Endothermic reactions.
Here are a few key aspects of these Endothermic Processes:
- Energy Absorption: Endothermic reactions require energy input in order to proceed; typically in the form of heat energy from their surroundings which is used to break chemical bonds within reactants and break reactions outward.
- Temperature Change: One distinguishing characteristic of endothermic reactions is their tendency to cause localized temperature drops due to energy being taken out from their environment and converted to cooling effects creating an immediate cooling effect.
- Examples: There are various examples of endothermic reactions including. Melting of Ice As solid ice absorbs heat energy from its surroundings, an endothermic reaction takes place which transforms it to liquid water and melts away.
- Evaporation of Water: Evaporating involves an endothermic reaction where heat energy from its surroundings is absorbed to generate enough energy for individual molecules of water to gain enough momentum to break their intermolecular bonds and become water vapor.
- Photosynthesis: Photosynthesis is an essential endothermic biochemical reaction found in plants that involve harnessing sunlight’s energy for the conversion of carbon dioxide and water to glucose and oxygen. It involves taking advantage of photon energy absorption.
- Electrolysis of Water: When an electric current is passed through water, an endothermic reaction takes place that uses energy from its source to separate hydrogen and oxygen gas molecules and release hydrogen as waste products.
- Role of Heat: Heat plays an essential part in endothermic reactions. It provides the energy needed to break bonds between reactants, leading to new bonds forming that eventually result in products. Applications and Real-World Examples: Endothermic reactions have many practical applications in everyday life and can be observed easily.
Cold Packs or Ice Packs utilize endothermic reactions for cooling purposes. When activated, these packs contain reactants that undergo endothermic reactions that absorb heat from their surroundings while simultaneously providing cooling effects. - Sports Injury Sprays: Sprays used to relieve muscle and joint pain rely on endothermic reactions when applied directly to the skin; when they absorb heat they cause endothermic reactions which provide cooling relief.
- Instant Cold Packs: These packs are commonly used as first aid treatments and as an immediate source of cooling. Contained within them are reactants which, once mixed together, undergo an endothermic reaction that quickly lowers the temperature.
Understanding endothermic reactions is invaluable across fields including chemistry, biochemistry, thermodynamics, and materials science. Understanding them enables scientists and researchers to design processes, create new materials, and explore energy transfer and conservation principles more easily.
Exothermic Reactions
Exothermic reactions provide such reactions to consider:
- Energy Release: Exothermic reactions involve the release of energy as part of their reaction. Usually in the form of heat but sometimes light or sound waves can also be released during such reactions.
- Temperature Change: A notable feature of exothermic reactions is their tendency to cause localized increases in temperature due to energy released during their reaction being distributed into their immediate surroundings and creating an intensive heating effect that heats it up further.
- Examples: There are various examples of exothermic reactions, including combustion reactions, When fuels such as wood or gasoline burn in air with oxygen present, exothermic reactions occur and release heat and light energy as part of this reaction chain.
- Neutralization Reactions: When acids react with bases such as hydrochloride acid and sodium hydroxide (NaOH), an exothermic reaction takes place that produces water while simultaneously dissipating heat energy. This produces water as a by-product while simultaneously dispersing any stored heat energy that has built up over time.
- Respiration: Cellular respiration in living organisms is exothermic and creates energy through the breakdown of glucose and other molecules essential to their cell’s proper function. Role of Heat: Heat is released during exothermic reactions when energy flows between reactant bonds and product bonds is less than their combined energies, leading to excess energy being converted to heat which raises temperatures around it.
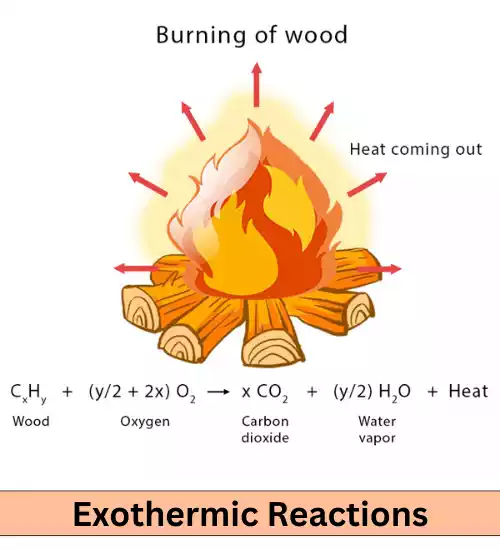
Understanding exothermic reactions is vitally important across various fields such as chemistry, thermodynamics, engineering, and environmental sciences. Understanding exothermic reactions enables scientists to design more energy-efficient energy conversion processes while creating safer handling protocols for reactive materials as well as examine energy transfer in chemical systems.
Comparison Chart of Endothermic and Exothermic Reactions
Sure! Here’s a comparison chart highlighting the differences between endothermic and exothermic reactions:
| Aspect | Endothermic Reactions | Exothermic Reactions |
|---|---|---|
| Energy | Absorb energy from the surroundings | Release energy to the surroundings |
| Temperature Change | Typically result in a decrease in temperature | Typically result in an increase in temperature |
| Energy Diagram | The reactants have lower energy than the products | The reactants have higher energy than the products |
| Examples | Photosynthesis, evaporation, melting | Combustion, oxidation, neutralization |
| Heat Flow | Heat flows into the system | Heat flows out of the system |
| Energy Transfer | Require an external source of energy | Energy is released during the reaction |
| Bond Breaking and Formation | Energy is required to break bonds, and more energy is released during bond formation | Energy is released during bond breaking and less energy is required for bond formation |
| Enthalpy Change | Positive enthalpy change (∆H > 0) | Negative enthalpy change (∆H < 0) |
| Reaction Profile | The energy of the products is higher than the energy of the reactants | The energy of the products is lower than the energy of the reactants |
| Examples | Photosynthesis, evaporation, melting | Combustion, oxidation, neutralization |
Remember that this chart provides a general overview, and there may be exceptions or additional details for specific reactions.
Factors Affecting Endothermic and Exothermic Reactions
Here are a few key variables that could have an impactful influence on such processes:
- The concentration of Reactants: Endothermic Reactions An increase in reactant concentration typically accelerates endothermic reactions as more reactant particles collide successfully, increasing collision chances and thus the absorption of energy by successful collisions and absorption mechanisms.
- Exothermic reactions: Reactant concentration typically does not have much of an impact on exothermic reactions, though reversible ones could possibly alter the equilibrium positions of reactions.
- Temperature and Pressure: Endothermic Reactions; By increasing either temperature or pressure, endothermic reactions typically increase at an increasing rate. Higher temperatures provide additional thermal energy which encourages breaking bonds while attenuating energy loss during energy absorption; higher pressure increases collision frequency among reactant particles increasing their chances of successful collision.
- Exothermic Reactions: Elevations in temperature or pressure tend to accelerate exothermic reactions. Higher temperatures provide additional thermal energy which speeds up reactions faster while higher pressure may increase collision frequency between reactant particles, increasing successful collision rates.
- Catalysts and Inhibitors: Catalysts are substances that accelerate chemical reactions without being consumed in their production process, providing alternative reaction pathways with lower activation energy that lower the barrier between energy inputs and reaction outcomes more readily resulting in quicker reaction rates and improved outputs.
- Inhibitors: Chemical reaction inhibitors can act to slow or halt chemical reactions by interfering with their mechanism or decreasing effective concentration levels of reactants. They may influence both endothermic and exothermic chemical processes by altering how reactions proceed and by decreasing effective concentration levels for reactants.
- Nature of Reactants: The type and composition of reactants involved can have an enormous effect on whether a reaction is endothermic or exothermic as different combinations of elements and compounds require different amounts of energy for bond-forming or breaking; chemical bonds determine energy changes during reaction processes.
Applications of Endothermic and Exothermic Reactions
Endothermic and exothermic reactions play an essential role across multiple fields and provide numerous applications.
Here are a few highlights of their significance:
- Energy Transfer and Conversion: mess Endothermic reactions play an essential part in energy storage and conversion processes. such as photosynthesis in plants where endothermic reactions convert sunlight to chemical energy in the form of glucose for storage in cells. Recognizing these endothermic processes helps develop renewable energy technologies as well as more effective storage systems. Exothermic reactions play a vital role in energy release and use, utilized by applications like combustion engines fossil-fuel power production plants, and heating systems. Their energy release serves practical needs that make these reactions essential parts of daily life.
- Industrial Applications: Knowledge of endothermic and exothermic reactions is crucial in chemical synthesis, manufacturing, and processing industries, such as automobile production. Understanding them allows engineers to optimize reaction conditions such as selecting suitable catalysts and controlling rate to increase productivity while improving yield and yield. Exothermic reactions have become ubiquitously utilized across industries like metallurgy, petrochemical, pharmaceuticals, and food processing to produce the energy needed to drive various chemical transformations and production processes.
- Environmental Impacts: Understanding energy changes associated with endothermic and exothermic reactions is integral for accurately evaluating environmental impacts, such as emissions from fossil fuel combustion releasing greenhouse gasses that contribute to climate change. Recognizing these reactions enables more sustainable forms of energy production while mitigating pollution issues.
- Safety Considerations: Understanding endothermic and exothermic reactions is vital in managing reactive materials safely, particularly exothermic ones which release energy rapidly, understanding this potential allows us to take precautionary steps against accidents or explosions occurring due to exothermic reactions. Endothermic reactions pose safety hazards if they involve dangerous materials or processes; to safeguard individuals and the environment from injury during these processes it is crucial that energy sources are handled with great care and effectively managed.
- Biological and Physiological Processes: Endothermic and exothermic reactions play an essential part in biological and physiological processes, for instance when living organisms digest their food, producing exothermic reactions that release energy for cell functions. Understanding such reactions contributes significantly to fields like biochemistry, physiology, and medicine.
- Scientific Research and Education: An Understanding of Endothermic and Exothermic Reactions is indispensable in Scientific Research and Education in fields as diverse as Chemistry, thermodynamics, and materials science. Understanding them allows scientists to better comprehend energy changes, Reaction Mechanisms as Well and Principles Pertaining to Energy Conservation and Transfer.
Conclusion
Understanding the Difference Between Endothermic and Exothermic Reactions is Integral for Understanding Energy Transformations that Take Place During Chemical Reactions. Endothermic Reactions Absorb Energy From Their Surroundings Producing Cooling Effects. Exothermic Reactions Release Energy back out into their Environments Leading to Heating Effects.
