Definition of Heat of Formation and Heat of Reaction
The heat of formation: The heat of formation, also known as enthalpy of formation, is the change in enthalpy that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1-atmosphere pressure). In simpler terms, it represents the amount of heat energy released or absorbed during the formation of a compound.
The heat of formation is usually measured in terms of energy per mole, such as kilojoules per mole (kJ/mol) or calories per mole (cal/mol). The measure of stability for compounds; its value indicates exothermic (heat is released during formation), while positive values indicate endothermic reactions (heat absorbed).
Calculating the heat of formation requires adding together each product’s heat of formation and subtracting that of each reactant, each multiplied by their respective stoichiometric coefficients.
This calculation is based on Hess’s law and utilizes tabulated values of heat of formation for individual elements and compounds.
The heat of formation is an important concept in chemistry as it helps determine the energy changes associated with chemical reactions. These tools can be applied across many fields, including thermodynamics and kinetics as well as design and optimization of chemical processes.
the heat of reaction: The heat of Reaction or Enthalpy of Reaction measures the change in enthalpy that takes place when chemical reactions take place. It represents the heat energy released or absorbed as the reactants are converted into products.
The heat of the reaction can typically be expressed as energy per mole, such as Kilojoules per Mole or Calories Per Mole (kJ/mol or Cal/mol). It indicates whether a reaction is exothermic (heat is released) or endothermic (heat is absorbed) based on the sign of the value.
The heat of the reaction can be determined experimentally by measuring the heat flow using calorimetry techniques. Alternatively, it can be calculated using the difference in heat of formation between the products and reactants, along with their stoichiometric coefficients.
This calculation is based on Hess’s law, which states that the total enthalpy change of a reaction is independent of the pathway taken.
Understanding and predicting the heat generated during chemical reactions are both necessary components of studying their thermodynamics. It provides valuable information about the energy changes associated with a reaction, its feasibility, and the conditions under which it is favorable.
This knowledge is essential in various applications, including chemical process design, energy storage, and understanding the behavior of substances under different conditions.
Importance of understanding the difference between the two concepts
Understanding the difference between heat of formation and heat of reaction is important for several reasons:
Energy Assessment: Heat of Formation and Heat of Reaction are both measures of energy changes in chemical processes. By differentiating between the two, chemists can accurately assess the energy requirements or releases associated with the formation of a compound versus the overall reaction.
Reaction Prediction: Knowledge of both heat of formation and heat of reaction aids in predicting the feasibility and directionality of chemical reactions. The heat of formation helps determine the stability and energy content of compounds, while the heat of reaction provides insights into the overall energy change during a reaction. These values can indicate whether a reaction is exothermic or endothermic and whether it is likely to proceed spontaneously.
Reaction Optimization: Understanding the difference between the two concepts allows chemists to optimize reactions for energy efficiency. By considering the heat of the formation of reactants and products, they can design reactions that minimize energy consumption or maximize energy release, leading to more efficient and sustainable processes.
Process Design: Heat of formation and heat of reaction is vital in the design of chemical processes. They help determine the heat transfer requirements, select appropriate reaction conditions, and assess the overall energy balance of a system. This information is crucial for designing safe, efficient, and economically viable chemical processes.
Comparative Analysis: Differentiating between heat of formation and heat of reaction enables chemists to compare different reactions or compounds on an energy basis. It provides a standardized approach to compare the energy content and stability of various compounds, aiding in the selection of suitable materials for specific applications.
Fundamental Understanding: Heat of formation and heat of reaction are fundamental concepts in thermodynamics and chemical kinetics. Grasping the distinction between the two enhances one’s understanding of energy changes in chemical systems, facilitating deeper insights into reaction mechanisms, equilibrium, and the interplay between energy and chemical transformations.
Comprehending the difference between heat of formation and heat of reaction is crucial for energy assessment, reaction prediction, reaction optimization, process design, comparative analysis, and fundamental understanding in the field of chemistry.
It enables chemists to make informed decisions, design efficient processes, and gain a deeper understanding of the energy dynamics involved in chemical reactions.
Heat of Formation
The heat of formation or the Enthalpy of Formation refers to the increase or decrease in Enthalpy produced when one mole of a compound is synthesized from its constituent elements in their initial states. It is a measure of the energy change associated with the formation of a compound.
The heat of Formation can often be expressed in terms of energy per mole. Common examples are Kilojoules per Mole (kJ/mol) and Calories per Mole (cal/mol). It can either be positive or negative, reflecting whether the formation of the compound occurs endothermically (heat absorbed), or exothermically (heat released).
The heat of formation depends upon various factors, including the arrangement and bonding of atoms within a compound, its physical state, and temperature and pressure conditions of formation.
The heat of formation is commonly determined experimentally using calorimetry techniques. It can also be calculated using Hess’s law, which states that the total enthalpy change of a reaction is independent of the pathway taken.
By using tabulated values of heat of formation for individual elements and compounds, along with their stoichiometric coefficients, the heat of formation of a compound can be calculated.
Heat of formation plays an integral part in many fields of chemistry, from thermodynamics and chemical kinetics, to process design. It provides valuable information about the stability and energy content of compounds, and it is used to predict the energy changes and feasibility of chemical reactions.
Furthermore, the heat of formation is employed in energy-related fields, such as combustion studies, fuel analysis, and the development of energy storage materials.
Calculation methods for the heat of formation
There are several calculation methods used to determine the heat of formation (enthalpy of formation) of a compound.
Here are the commonly employed methods:
Hess’s Law: Hess’s law states that the total enthalpy change of a reaction is independent of the pathway taken. By using known enthalpy values of other reactions, the heat of the formation of a compound can be calculated. The method involves manipulating the given reactions and their enthalpy values to cancel out intermediate compounds and obtain the desired compound. Then, the sum of the enthalpy changes of the individual reactions gives the heat of the formation of the compound.
Bond Enthalpy: This method is based on the concept that the enthalpy change in a reaction is determined by the breaking and forming of chemical bonds. Calculating heat of formation involves considering the difference between reactant bond enthalpies and product bond enthalpies; The values used for bond enthalpies are typically tabulated experimental or theoretical values.
Group Additivity: Group additivity methods use the concept that the heat of formation of a compound can be estimated by summing the contributions of its constituent functional groups or molecular fragments. Each functional group or fragment is assigned a known group increment value, which represents the change in heat of formation associated with that group. By summing up the increments for all the groups in the compound, the overall heat of formation can be estimated.
Computational Methods: With advancements in computational chemistry, various quantum chemical methods, such as density functional theory (DFT) and ab initio calculations, can be employed to predict the heat of the formation of a compound. These methods involve solving the Schrödinger equation to determine the electronic structure and energy of the compound. Computational approaches are particularly useful when experimental data is limited or unavailable.
Be mindful that heat of formation calculations may differ significantly depending on which approach and level of approximation is taken to produce them. Experimental measurements are generally considered more reliable, but computational methods can provide valuable estimates when experimental data are scarce.
Heat of Reaction
The heat of reaction or the enthalpy of reaction refers to any change in energy that takes place as part of chemical reactions. It represents the heat energy released or absorbed as the reactants are converted into products.
The heat of the reaction can typically be expressed as energy per mole. Common units for measuring this include Kilojoules per Mole (kJ/mol) or Calories Per Mole (cal/mol). It indicates whether a reaction is exothermic (heat is released) or endothermic (heat is absorbed) based on the sign of the value.
The heat of the reaction can be determined experimentally by measuring the heat flow using calorimetry techniques. Calorimeters are used to accurately measure the heat changes associated with a reaction, allowing for the determination of the heat of the reaction.
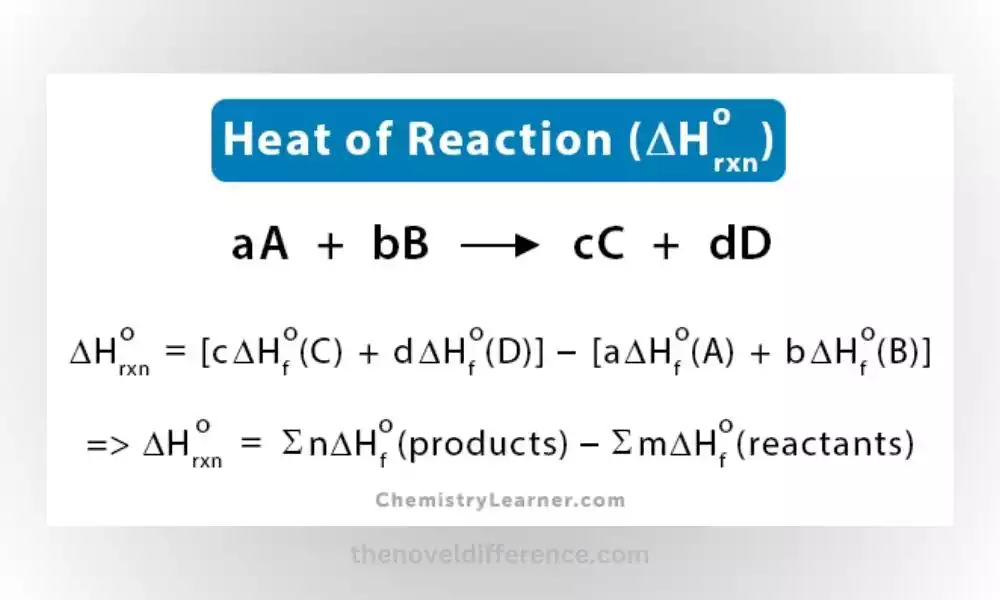
Alternatively, the heat of the reaction can be calculated using the difference in heat of formation between the products and reactants, along with their stoichiometric coefficients. This calculation is based on Hess’s law, which states that the total enthalpy change of a reaction is independent of the pathway taken.
The heat of reaction is an integral concept in thermodynamics as it provides insight into energy changes associated with chemical reactions. Assessing potential reactions helps identify their feasibility, directionality, and likely conditions of occurrence.
Understanding the heat of a reaction is crucial for various applications, including chemical process design, energy storage, and the study of reaction kinetics. It allows chemists and engineers to optimize reaction conditions, design efficient processes, and assess the energy balance of chemical systems.
Furthermore, the heat of reaction plays a significant role in understanding the behavior of substances under different conditions and in the development of energy conversion technologies.
Calculation methods for the heat of reaction
There are several methods used to calculate the heat of reaction (enthalpy of reaction) for a chemical reaction.
Here are some commonly employed calculation methods:
Hess’s Law: Hess’s law states that the total enthalpy change of a reaction is independent of the pathway taken. Using this principle, the heat of the reaction can be calculated by considering a series of known reactions and their enthalpy changes. By manipulating and combining these reactions appropriately, the desired reaction can be obtained, and the sum of the enthalpy changes of the individual reactions gives the heat of the reaction.
Bond Enthalpy: The bond enthalpy method utilizes the concept that the enthalpy change in a reaction is determined by the breaking and forming of chemical bonds. By taking into account the difference between reactant bond enthalpies and product bond enthalpies, the heat of the reaction can be determined. Bond enthalpy values can be obtained from experimental or theoretical data.
Standard Enthalpy of Formation: The standard enthalpy of formation method involves calculating the heat of the reaction based on the standard enthalpy of formation values of the reactants and products. The standard enthalpy of formation is the enthalpy change when one mole of a compound is formed from its elements in their standard states. By subtracting the sum of standard enthalpies of reactant formation from that of product formation, the heat of the reaction can be determined.
Computational Methods: With the advancements in computational chemistry, various quantum chemical methods, such as density functional theory (DFT) and ab initio calculations, can be employed to predict the heat of a reaction. These methods involve solving the Schrödinger equation to determine the electronic structure and energy of the reactants and products. Computational approaches can provide valuable estimates of the heat of reaction when experimental data is limited or unavailable.
Notably, it should be remembered that heat of reaction calculations may differ significantly depending on the method and degree of approximation employed.
Experimental measurements are generally considered more reliable, but computational methods can provide valuable estimates when experimental data is scarce or when exploring reactions that are difficult to measure experimentally.
Differences Between Heat of Formation and Heat of Reaction
The differences between heat of formation and heat of reaction can be summarized as follows:
Definition and Scope:
Heat of Formation: Heat of formation refers to the energy change incurred when one mole of an organic compound is formed from its constituent elements in their standard states. It specifically focuses on the formation of a compound.
Heat of Reaction: “Heat of Reaction” refers to any energy change occurring during chemical reactions. It encompasses the overall energy change associated with the conversion of reactants into products.
Calculation:
Heat of Formation: The heat of formation is typically determined using experimental measurements or calculated based on known values of heat of formation for elements and compounds. Subtracting the heat needed for reactant formation from that required for product formation can provide an indication of where any inefficiencies occur in a chemical process.
Heat of Reaction: Experimentally measuring heat flow during reaction using calorimetry can allow one to ascertain its heat content and ultimately its heat of reaction. It can also be calculated by considering the difference in the enthalpy of the products and reactants, as obtained from experimental data or theoretical calculations.
Energy Focus:
Significance:
Heat of Formation: Heat of formation is important for understanding the energy content and stability of compounds. It is used in various fields, including thermodynamics, kinetics, and process design.
Heat of Reaction: Heat of reaction is crucial for determining the energy changes, feasibility, and directionality of chemical reactions. It is valuable in process optimization, energy storage, and the study of reaction kinetics.
Units:
Heat of Formation: When discussing heat of formation, typically measured in terms of energy per mole. For instance, this might include units like Kilojoules per Mole (kJ/mol) or Calories per Mole (cal/mol).
Heat of Reaction: Reactant heat may also be expressed in terms of energy per mole, for instance, kilojoules per mole or caloric equivalent per mole.
The key differences between heat of formation and heat of reaction lie in their definitions, calculation methods, energy focus, significance, and scope. The heat of formation specifically pertains to the formation of a compound, while the heat of reaction encompasses the overall energy change during a chemical reaction.
Both concepts provide important insights into energy changes in chemical systems and are utilized in various areas of chemistry and process engineering.
Similarities between Heat of Formation and Heat of Reaction
There are various differences between heat of formation and reaction; however, they also share many similarities. These similarities include:
Energy Quantification: Both heat of formation and heat of reaction involves the quantification of energy changes associated with chemical processes. They provide a measure of the energy involved in the formation of compounds or the overall energy change during a reaction.
Enthalpy Measurements: Both concepts are related to the measurement of enthalpy, which is a thermodynamic property representing the heat content of a system at constant pressure. The enthalpy change is determined for both heat of formation and heat of reaction calculations.
Units of Measurement: When discussing both heats of formation and reaction, its energy equivalent per mole is often expressed in units such as kilojoules per mole (kJ/mol) or calories per mole (cal/mol). This allows for standardized comparisons and calculations.
Hess’s Law Application: Both concepts rely on the principle of Hess’s law, which states that the total enthalpy change of a reaction is independent of the pathway taken. Hess’s law is utilized in the calculation of both the heat of formation and the heat of reaction.
Thermodynamic Considerations: Both heat of formation and heat of reaction are important in the study of thermodynamics and energy changes in chemical systems. They provide information about the stability, energy content, and feasibility of reactions, aiding in the understanding of chemical processes.
While there are differences in the specific focus and calculations for the heat of formation and heat of reaction, their underlying principles and applications within thermodynamics and energy analysis make them related concepts in the field of chemistry.
Side-by-Side Comparison – Heat of Formation vs Heat of Reaction in Tabular Form
Here is a side-by-side comparison of the key aspects of heat of formation and heat of reaction:
| Aspect | Heat of Formation | Heat of Reaction |
|---|---|---|
| Definition | Change in enthalpy when one mole | Change in enthalpy during a chemical |
| of a compound is formed from its | reaction | |
| constituent elements | ||
| Calculation | Using experimental measurements | Measured experimentally or calculated |
| Methods | or calculated based on known | using enthalpy changes of reactants and |
| values of heat of formation | products | |
| Energy Focus | Formation of a compound | Overall energy change in a reaction |
| Significance | Stability and energy content of | Feasibility, directionality, and energy |
| compounds | requirements of chemical reactions | |
| Units | kJ/mol, cal/mol, etc. | kJ/mol, cal/mol, etc. |
| Hess’s Law Application | Used in calculations to determine | Used to calculate the heat of reaction |
| the heat of the reaction | based on known enthalpies | |
Note that although the heat of formation and reaction represent two different ways of looking at energy changes associated with chemical processes, they both serve as valuable ways of conceptualizing how they operate.
Conclusion
Understanding the difference between heat of formation and heat of reaction is crucial in the field of chemistry. The heat of formation focuses on the energy change when a compound is formed from its constituent elements, while the heat of reaction encompasses the overall energy change during a chemical reaction.
Both concepts involve quantifying energy changes and are measured in units of energy per mole. They utilize principles such as Hess’s law and are important in thermodynamics, kinetics, and process design. Understanding these concepts allows for predicting the feasibility, directionality, and energy requirements of chemical reactions.
Calculation methods for the heat of formation include Hess’s law, bond enthalpy, and group additivity. The heat of the reaction can be determined experimentally using calorimetry or calculated based on the difference in enthalpy of the reactants and products.
