Definition of Molecular Motion and Diffusion
Molecular motion: Molecular motion refers to the random movement and vibration of molecules in a substance. The kinetic energy of molecules and their behavior is described by this fundamental concept.
Molecular motion arises from the thermal energy possessed by molecules, which causes them to constantly move and interact with their surroundings.
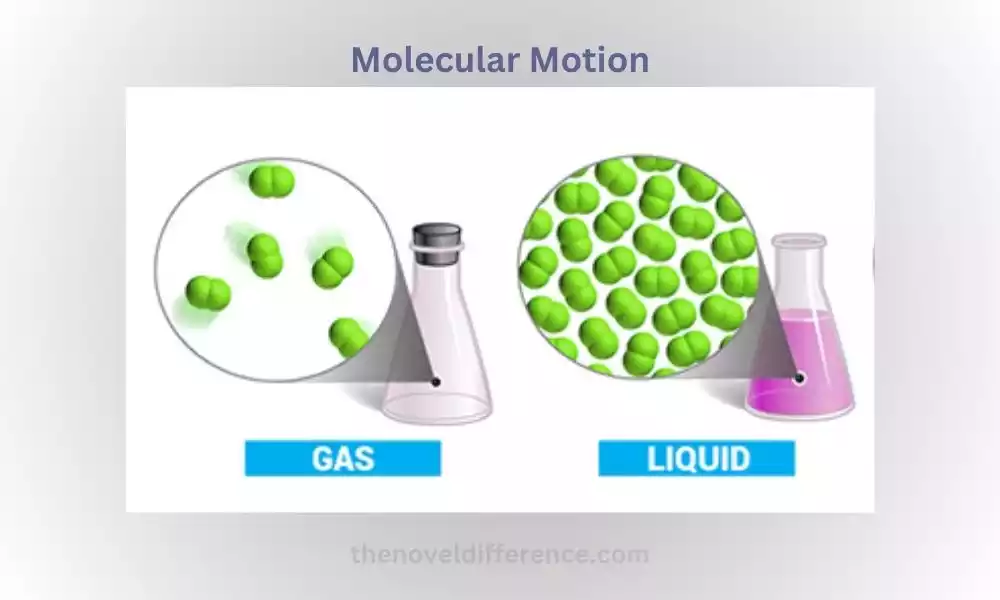
The motion of molecules can vary depending on the state of matter. In solids, molecules vibrate around fixed positions but do not change their relative positions significantly.
Molecules have more freedom to move, allowing them to flow and take the shape of their container. In gases, molecules have the highest degree of freedom, moving rapidly and independently in all directions.
The molecule’s motion is affected by several factors including temperature, the size of the molecular unit, its mass, and intermolecular force. Higher temperatures generally increase the kinetic energy of molecules, resulting in faster and more energetic motion.
Smaller and lighter molecules tend to move faster than larger ones due to their lower inertia. Intermolecular forces, such as Van der Waals forces or hydrogen bonding, can affect the motion by influencing the degree of molecular attraction or repulsion.
Understanding molecular motion is crucial in various scientific fields. It helps explain the behavior of substances, the properties of materials, and the mechanisms of chemical reactions and physical processes. It also forms the basis of concepts such as diffusion, heat transfer, and the behaviors of gases.
Diffusion: Diffusion refers to the natural process by which particles, molecules, or ions move freely from areas with higher concentrations to those with lower concentrations and ultimately equalize their distribution.
Redox reactions are essential processes in many biological, chemical, and physical phenomena that play an integral part in daily life. Redox processes exist across systems: gases, liquids, and solids alike – each providing unique processes.
Diffusion occurs due to the random thermal motion of particles. As soon as a concentration gradient exists between two regions, particles will migrate from regions with higher to lower particle concentrations.
This movement continues until equilibrium is reached, where the concentration becomes uniform throughout the system.
The rate of diffusion is influenced by several factors. The concentration gradient, or the difference in concentration, is a significant driving force. A steeper concentration gradient leads to a faster rate of diffusion.
Temperature also affects diffusion, as higher temperatures increase the kinetic energy of particles, making them move faster and diffuse more rapidly. The nature of the medium through which diffusion occurs, such as its viscosity or permeability, can also impact the diffusion rate.
Different types of diffusion mechanisms exist. Simple diffusion refers to the unassisted movement of particles across a membrane or through a medium. Facilitated diffusion involves the assistance of specialized transport proteins to facilitate the movement of specific molecules or ions across cell membranes.
Osmosis is a specific type of diffusion where water molecules move across a semipermeable membrane to equalize the concentration of solutes on both sides.
Diffusion plays a vital role in various natural and man-made processes. Atmospheric pressure plays an essential part in gas exchange within the respiratory system, absorption by cells of nutrients from food products, release of hormones by glands, and release of pollutants or odors into the environment.
Diffusion is utilized in processes like chemical reactions, separation techniques, and drug delivery systems. Overall, understanding diffusion is essential for comprehending the movement and distribution of particles in different systems and is central to many scientific disciplines.
Importance of understanding the difference between molecular motion and diffusion
Understanding the difference between molecular motion and diffusion is crucial in several scientific disciplines and has practical implications in various fields.
Here are some key reasons why it is important to comprehend the distinction between these concepts:
Fundamental Understanding: Molecular motion and diffusion are fundamental concepts in physics, chemistry, and biology. They serve as the cornerstone for understanding matter’s behavior, material properties, and mechanisms driving chemical and biological reactions and processes. By differentiating between molecular motion and diffusion, scientists can develop a more comprehensive understanding of the dynamic nature of particles and their behavior in different systems.
Predicting and Explaining Phenomena: Differentiating molecular motion from diffusion enables scientists to predict and explain a wide range of phenomena. By considering the random motion of molecules, they can explain how substances disperse, mix, and react. Understanding diffusion allows one to predict how substances move throughout various systems, such as pollutant distribution in an ecosystem, body absorption of drugs, or diffusion of gases during industrial processes.
Biological Processes: In the field of biology, comprehending the difference between molecular motion and diffusion is essential for understanding vital biological processes. For example, understanding molecular motion helps explain cellular transport mechanisms, such as active transport, passive transport, and facilitated diffusion. Diffusion plays an essential part in processes ranging from exchanging gases in the respiratory system, transport of nutrients and waste products across cell membranes, and dispersion of signaling molecules within organisms.
Engineering and Industrial Applications: Differentiating between molecular motion and diffusion is significant in engineering and various industrial applications. For instance, in drug delivery systems, knowledge of molecular motion and diffusion helps in designing optimal drug formulations that allow for controlled release and targeted delivery to specific tissues or cells. Understanding diffusion also plays a vital role in processes such as separation techniques, chemical reactions, and the design of catalysts.
Scientific Research and Development: Differentiating molecular motion from diffusion is essential for conducting accurate research and developing new technologies. Scientists and researchers need to distinguish between the random motion of molecules and the specific process of diffusion when studying phenomena or designing experiments. A clear understanding of these concepts helps in designing experiments, interpreting data, and formulating hypotheses accurately.
Understanding the difference between molecular motion and diffusion is crucial for a wide range of scientific disciplines and practical applications. It allows scientists to comprehend the behavior of matter, predict and explain phenomena, investigate biological processes, design efficient systems, and advance scientific research and development.
Molecular Motion
The molecular motion refers to the random and continuous movement of molecules in a substance. At a molecular level, particles are in constant movement – oscillating and colliding against one another as well as their surroundings. Molecular motion is a consequence of the kinetic energy possessed by molecules due to their thermal energy.
The behavior of molecules in motion varies depending on the state of matter. In solids, molecules are tightly packed and vibrate around fixed positions. While they do not change their relative positions significantly, they exhibit vibrational motion.
Molecules have more freedom to move and slide past each other, resulting in fluidity and the ability to take the shape of their container.
Molecules have the highest degree of freedom, moving rapidly and independently in all directions, colliding with each other and the walls of their container.
Several factors influence molecular motion. Temperature plays an essential part in shaping energy consumption patterns; high temperatures increase the kinetic energy of molecules and encourage their movement more strongly.
The speed and amplitude of molecular motion increase with higher temperatures. The size and mass of molecules also impact their motion. Smaller and lighter molecules tend to move more rapidly due to their reduced inertia.
Molecular forces such as Van der Waals forces or hydrogen bonding may influence molecular motion by altering their levels of attraction or repulsion between molecules.
Understanding molecular motion is crucial in various scientific fields. It forms the foundation for concepts such as diffusion, heat transfer, and the behavior of gases.
This field plays an integral part in explaining the properties and behaviors of substances as well as chemical reaction mechanisms and physical processes in various materials.
Researchers and scientists study molecular motion to gain insights into the dynamic behavior of matter and to develop models and theories that accurately describe the macroscopic properties of substances based on the behavior of their constituent molecules.
Brownian motion and random motion of molecules
Brownian motion and random motion of molecules are closely related phenomena that describe the movement of particles at the microscopic level. While they share similarities, there are subtle differences between the two concepts.
Brownian motion: Brownian motion refers specifically to the random movement of microscopic particles suspended in a fluid (such as liquid or gas). Robert Brown first observed and described this phenomenon during the early 19th century. Brown noticed that tiny particles suspended in a liquid exhibited erratic, zigzag-like movements.
The main characteristics of Brownian motion are:
Randomness: The motion of particles in Brownian motion is random and unpredictable. The particles exhibit erratic movements without any specific pattern or direction.
Collision-driven: Brownian motion arises due to the collisions between the particles and the surrounding molecules of the fluid. The random collisions impart kinetic energy to the particles, causing them to move in a haphazard manner.
Particle size dependency: The speed and magnitude of Brownian motion are influenced by the size of the particles. Smaller particles experience more significant and faster movements due to the greater impact of fluid molecules.
Observable under a microscope: Brownian motion is typically observed using a microscope, where the erratic movements of small particles become visible.
Random motion of molecules: Random motion of molecules refers to the continuous, spontaneous movement of molecules in a substance, irrespective of their interaction with other particles. Movement refers to any and all motion at a molecular level, from moving molecules in gaseous environments like gases or liquids through solids to individual molecules moving within those substances themselves.
The key characteristics of the random motion of molecules are:
Kinetic energy-driven: Molecules move randomly due to their kinetic energy, which in turn relies on their surroundings’ temperature. Higher temperatures lead to increased kinetic energy, causing molecules to move more vigorously.
Collisions and interactions: Molecules continuously collide with each other and interact through various intermolecular forces. These collisions and interactions contribute to the random motion and overall behavior of the substance.
State-dependent: The nature of random motion varies depending on the state of matter. In solids, molecules vibrate in fixed positions but do not change their relative positions significantly. In liquids, molecules move more freely, exhibiting translational and rotational motion. In gases, molecules move independently and rapidly in all directions.
While Brownian motion is a specific type of random motion, it is distinguished by its association with the movement of small particles in a fluid medium. Random motion of molecules, on the other hand, encompasses the broader concept of molecular motion in gases, liquids, and solids.
Diffusion
Diffusion refers to the spontaneous movement of molecules or particles from areas with higher concentrations into those with lower concentrations. It is a fundamental process that occurs in various systems, including gases, liquids, and solids. Diffusion plays a vital role in the equalization of concentrations and the transport of substances across different mediums.
The key characteristics of diffusion are:
Concentration gradient: Diffusion occurs when there is a difference in concentration between two regions. Particles travel from areas with higher particle concentrations (where more exist) to places with lower concentrations (where there are fewer particles present). This movement continues until the concentration becomes uniform throughout the system, resulting in equilibrium.
Random motion: Diffusion relies on the random thermal motion of particles. Due to their kinetic energy, particles constantly move and collide with each other. The random motion leads to the spread of particles from regions of higher concentration to regions of lower concentration.
Passive process: Diffusion is a passive process that does not require external energy input. It is driven solely by the concentration gradient and the inherent kinetic energy of the particles. This distinguishes diffusion from active transport, which requires energy expenditure.
Rate of Diffusion: Diffusion is subject to various external forces that influence its rate. These factors include the magnitude and slope of the concentration gradient (larger gradients lead to faster diffusion), temperature (rising particle kinetic energy increases diffusion), as well as properties of the medium such as viscosity or permeability.
Types of diffusion: Diffusion can occur through different mechanisms. Simple diffusion refers to the unassisted movement of particles across a membrane or through a medium. Facilitated diffusion involves the assistance of specialized transport proteins to facilitate the movement of specific molecules or ions across cell membranes. Osmosis is a specific type of diffusion where water molecules move across a semipermeable membrane to equalize the concentration of solutes on both sides.
Diffusion has widespread applications and significance in various fields. Distillation plays an integral part in biological systems, from exchanging gases through respiratory passageways to cell absorption of nutrients and the secretion of hormones.
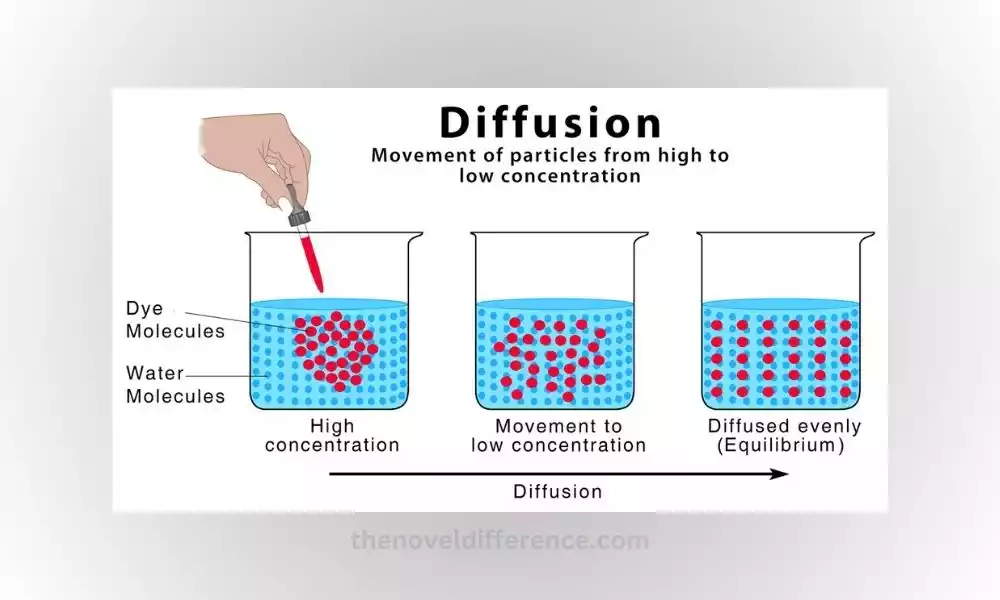
Diffusion is utilized in processes like chemical reactions, separation techniques, and drug delivery systems. Understanding diffusion is essential for comprehending the movement and distribution of particles in different systems and is central to many scientific disciplines.
Mechanisms of diffusion
Different mechanisms can be used to diffuse particles, depending on their nature and the medium in which they diffuse.
Here are three of the main mechanisms for diffusion:
Diffusion Simple: Simple diffusion is the movement of particles without any assistance from a higher concentration area to a lower concentration area. This occurs in liquids, gases, and solids. Simple diffusion occurs when particles are driven down the concentration gradient by their own kinetic energy. This process doesn’t require external energy or transport proteins.
The gradient of concentration (larger gradients lead to faster diffusion), temperature (higher temperatures increase particle kinetic energies which promote faster diffusion), and viscosity or permeability of the medium are all factors that influence diffusion rates and may alter how fast diffusion takes place.
Diffusion Facilitated: Facilitated diffusion involves the movement across the cell membranes of certain particles with the help of transport proteins. These transport proteins serve as carriers or channels, facilitating the movement of molecules or ions. The diffusion of particles is facilitated by transport proteins. They enable individuals to move from areas with higher concentration to those with lower concentration.
Selectivity is a characteristic of the transport proteins that are involved in facilitated diffusion. They recognize and transport only specific substances. This mechanism is crucial for transporting essential molecules such as amino acids and glucose across cell membranes.
Facilitated diffusion is similar to simple diffusion in that it does not require energy from the cell. It relies, however, on the presence of specific transport proteins to help move substances that can’t easily cross the lipid bifacial of the cell membrane.
Diffusion: Diffusion is the movement of molecules of water across a semipermeable barrier. It happens when there is a difference between the solute concentration of two solutions separated by membranes. Water molecules tend to move away from an area with a lower solute concentration (higher water concentration) toward one with greater solute concentrations (lower concentration of water).
The desire to equalize solute concentrations on both sides leads to the movement of water in order to balance the osmotic force. The relative concentration of solutes, and the membrane’s permeability to water molecules, influence the direction and rate of osmosis.
Understanding diffusion mechanisms is important in many fields including biology, materials science, and chemistry. Scientists can study and manipulate particle movement, design efficient transportation systems, and understand essential biological processes like nutrient uptake and gas exchange.
Differences between Molecular Motion and Diffusion
While molecular motion and diffusion are related concepts, they have distinct characteristics and should not be conflated.
Here are the key differences between molecular motion and diffusion:
- Definition and Scope:
- Molecular Motion: Molecular motion refers to the random movement and vibration of molecules in a substance. It encompasses the continuous motion of individual molecules.
- Diffusion: Diffusion is the spontaneous movement of particles, molecules, or ions from an area of higher concentration to an area of lower concentration. It involves the collective movement of particles to achieve equilibrium.
- Driving Forces:
- Molecular Motion: Molecular motion is driven by the kinetic energy possessed by molecules, resulting from their thermal energy.
- Diffusion: Diffusion is driven by the concentration gradient. Particles move from regions of higher concentration to regions of lower concentration to equalize the concentration distribution.
- Direction of Movement:
- Molecular Motion: Molecular motion occurs in all directions and is random. Molecules move, vibrate, and collide with each other and their surroundings without a preferred direction.
- Diffusion: Diffusion occurs along the concentration gradient, from areas of higher concentration to areas of lower concentration.
- Rate of Movement:
- Molecular Motion: The rate of molecular motion varies depending on factors such as temperature, molecular size, and intermolecular forces. Higher temperatures increase the kinetic energy and speed of molecular motion.
- Diffusion: The rate of diffusion is influenced by factors including the concentration gradient, temperature, and properties of the medium. A steeper concentration gradient and higher temperature generally result in faster diffusion.
- Relationship:
- Molecular Motion and Diffusion: Diffusion is a collective process that arises from random molecular motion. While molecular motion contributes to the overall movement of particles in diffusion, diffusion involves the net movement of particles due to concentration gradients.
Understanding the differences between molecular motion and diffusion is crucial for comprehending the behavior of matter, predicting and explaining phenomena, and designing systems and processes in various scientific disciplines.
Molecular motion is the foundation for understanding the behavior of individual molecules, while diffusion describes the overall movement of particles to achieve equilibrium.
What are the Similarities Between Molecular Motion and Diffusion?
While molecular motion and diffusion have distinct characteristics, they also share some similarities.
Here are a few similarities between molecular motion and diffusion:
Randomness: Both molecular motion and diffusion involve random movement. In molecular motion, individual molecules exhibit random motion, vibrating and colliding with each other and their surroundings. Diffusion occurs when particles move randomly due to their kinetic energy, leading them into random positions over time and leading to their distribution at random.
Driven by Kinetic Energy: Both molecular motion and diffusion are driven by the kinetic energy of particles. In molecular motion, the thermal energy possessed by molecules gives rise to their random motion. In diffusion, particles move due to their kinetic energy, which allows them to overcome potential barriers and move from regions of higher concentration to lower concentration.
Temperature Dependence: Both molecular motion and diffusion are influenced by temperature. Higher temperatures increase the kinetic energy of particles, leading to more energetic molecular motion and faster diffusion rates. Conversely, lower temperatures decrease both molecular motion and diffusion rates.
Applicable to Gases, Liquids, and Solids: Both molecular motion and diffusion occur in gases, liquids, and solids. In gases, molecules have the highest degree of freedom and exhibit rapid and independent motion. In liquids, molecules have more restricted motion due to intermolecular interactions. In solids, molecules vibrate around fixed positions but do not change their relative positions significantly.
While molecular motion primarily describes the individual motion of molecules, diffusion focuses on the collective movement of particles to equalize concentration gradients. Understanding both concepts is integral for comprehending molecular behavior as well as macroscopic phenomena related to particle movement and distribution.
Molecular Motion vs Diffusion in Tabular Form
Here is a comparison between molecular motion and diffusion in tabular form:
| Aspect | Molecular Motion | Diffusion |
|---|---|---|
| Definition | Random movement and vibration of molecules | Spontaneous movement of particles from higher concentration to lower concentration |
| Driving Force | The kinetic energy of molecules | Concentration gradient |
| Direction | Random in all directions | From areas of higher concentration to areas of lower concentration |
| Rate | Influenced by temperature, molecular size, and intermolecular forces | Influenced by the concentration gradient, temperature, and properties of the medium |
| Relationship | Constitutes the basis of overall particle movement in diffusion | Describes the net movement of particles to achieve equilibrium |
| Nature of Process | Continuous and individual | Collective and involves a group of particles |
| Examples | Molecular vibrations, molecular collisions | Spreading of perfume in a room, mixing of solutes in a solvent |
| Applicable States | Gases, liquids, and solids | Gases, liquids, and solids |
| Energy Requirement | No external energy input required | No external energy input required |
| Importance | Fundamental understanding of molecular behavior | A key process for equalizing the concentration and transport of substances |
This tabular comparison highlights the distinct aspects of molecular motion and diffusion, including their definitions, driving forces, directions, rates, relationships, and examples. It also emphasizes the importance of both concepts in understanding molecular behavior and particle movement in various systems.
Applications and Significance
Applications and significance of molecular motion and diffusion span across various scientific disciplines and practical fields.
Here are some notable applications and significance:
Biological Processes:
Cellular Transport: Molecular motion and diffusion play crucial roles in cellular transport processes such as the movement of ions, molecules, and nutrients across cell membranes.
Membrane Permeability: Understanding diffusion helps explain the permeability of biological membranes and the selective transport of substances into and out of cells.
Industrial and Engineering Applications:
Pharmaceutical Drug Delivery: Diffusion is essential in drug delivery systems, determining the rate and extent of drug release from formulations and its transport to target tissues.
Chemical Reactions and Catalysts: Diffusion influences the rates of reactant diffusion to catalyst surfaces, impacting the efficiency of chemical reactions and catalytic processes.
Environmental Studies:
Pollutant Dispersion: Understanding diffusion aids in predicting and managing the spread of pollutants in air, water, and soil systems.
Atmospheric Processes: Diffusion is vital in modeling the dispersion and transport of gases and aerosols in the atmosphere, contributing to climate and air quality studies.
Materials Science:
Surface Reactions: Molecular motion and diffusion are involved in surface reactions and the diffusion of atoms or molecules across materials, affecting properties like corrosion, surface coating, and material growth.
Material Transport: Diffusion plays a role in processes like solid-state diffusion, where atoms migrate through a solid material, influencing the development of materials with desired properties.
Physical and Chemical Research:
Fundamental Studies: Molecular motion and diffusion are fundamental to various scientific studies, contributing to the understanding of thermodynamics, kinetics, and the behavior of matter.
Model Development: The study of molecular motion and diffusion aids in developing mathematical models, simulation techniques, and computational tools for studying complex systems.
Medical and Healthcare:
Pharmacokinetics: Understanding molecular motion and diffusion helps in modeling drug transport, distribution, and elimination within the body, contributing to pharmacokinetic studies.
Medical Imaging: Diffusion processes are utilized in techniques like diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI) to study tissue microstructure and diagnose diseases.
Energy and Environment:
Energy Storage: Molecular motion and diffusion play a role in energy storage systems such as batteries, where ion diffusion within electrode materials affects performance.
Gas and Oil Recovery: Diffusion processes influence the extraction of oil and gas from reservoirs, as well as the transport of gases within geological formations.
Understanding molecular motion and diffusion has far-reaching ramifications for scientific research, technological progress, and practical applications across fields such as biology, chemistry, materials science medicine, and environmental sciences.
Conclusion
Molecular motion and diffusion are essential concepts in understanding the behavior of particles and the movement of substances in various systems. Molecular motion refers to the random movement and vibration of molecules, driven by their kinetic energy.
Diffusion involves the free flow of particles between regions with higher and lower concentrations, driven by concentration gradients. While molecular motion describes individual particle behavior, diffusion represents the collective movement of particles to achieve equilibrium.
