Introduction of Spectrochemical and Nephelauxetic Series
In the world of coordination chemical chemistry, The Spectrochemical, as well as the Nephelauxetic Series, are two fundamental concepts that aid in understanding the behavior of metal ions as well as the ligands within coordination complexes.
These series are crucial to explain the color, magnetic properties, and stability of the coordination compound. This Spectrochemical Series is primarily concerned with the ligands’ order based on their capability to break D-orbitals, which affects the absorption spectra of compounds.
Contrastingly, the Nephelauxetic Series focuses on the binding ligand’s capability to increase the covalency between metal and ligand. Together they are crucial to discovering the intricate details of coordination chemistry. They assist scientists in the development and understanding of complex chemical compounds.
What is a Spectrochemical series?
A spectrum chemical series is a classification or order of ligands according to their capacity to alter their electronic characteristics of complexes made up of transition metals. It gives insight into the ways that different ligands alter the absorption spectrum and the colors that are observed in complexes of metals. The placement of ligands in the spectrochemical sequence helps to identify the arrangement and splitting of d orbitals, in the presence of the ligands that directly affect the electronic transitions and consequent hues of metal complexes.
The spectrochemical series takes into consideration a variety of factors that affect the influence of the ligand on the electronic structure, like how charged the metal is the size and nature of the ligand, as well as the oxidation status that the metal is in. The ligands that have powerful interactions with metal and trigger a significant split in the orbitals of the d are placed further up in the series in contrast, ligands that exhibit less interaction and smaller splits are positioned lower in the series.
The spectrochemical sequence is useful in understanding and predicting the absorption and colors that are characteristic of the transition metal compounds. It aids scientists and chemists in picking the appropriate ligands to use for specific purposes, like developing dyes, pigments, and other materials that have desirable optical characteristics. Through the study of the spectrochemical series, scientists can make educated decisions regarding the ligand-metal interaction and electronic changes that take place throughout complex processes, helping the creation of new materials as well as advancing the understanding and application of coordination chemical reactions.

What is the Nephelauxetic series?
A nephelauxetic sequence is an arrangement of ligands or metal ions that are ordered in accordance with their nephelauxetic effects. This term is used primarily to describe the transition metal ions. The term”nephelauxetic” is used to describe a reduction in the reach interelectronic repel parameter.
A reduction in any parameter is a sign of less repulsion between two electrons within the d-orbitals of a metallic and also that the orbital is bigger within the complex. This is known as electron cloud expansion in the complex and is vital to determine the magnitude of the nephelauxetic effects.
If ligands are placed in an order according to the effects of nephelauxetic, it is close to the spectrochemical sequence. However, the arrangement illustrates the capacity of compounds to create covalent bonds with metal ions. The ligands on the left side have a lower chance of creating a covalent bond with metal ions, whereas those on the right have a larger impact.
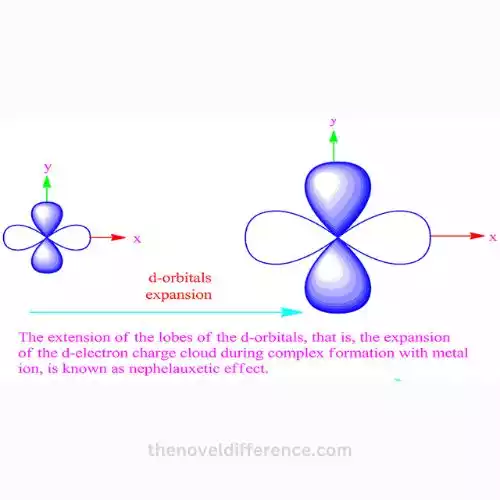
Differences between Spectrochemical and Nephelauxetic Series
The Spectrochemical and Nephelauxetic Series are two different concepts that are used to explain the interactions of ligands with complexes of transition metals.
Below are the most important distinctions between the Spectrochemical Series as well as the Nephelauxetic Series:
Scope and the focus:
The spectrum of spectrochemical studies focuses on the effects of ligands on electron properties, absorption spectra, and the colors of complexes made up of transition metals. It gives insight into the ways that different ligands alter the configuration and splitting of d orbitals. It also aids in predicting the observed absorption spectra and colors that the structures.
Nephelauxetic Series: The Nephelauxetic Series is focused on the impact of the ligand in the energy field of the d orbitals within the transition-metal complexes. It determines the extent to the degree that a ligand weakens or decreases the energy of d orbitals because of their field force. It aids in predicting the extent to which ligand field splitting occurs as well as the crystal field stabilization energy (cfse) of the complexes.
Factors considered:
The spectrochemical series considers aspects like the size, charge, and oxidation state. It analyzes how these elements influence the interaction between the ligand and the metal ion, and the resultant splitting of the orbitals with d.
Nephelauxetic series: Nephelauxetics is primarily focused on the ligand field strength as well as its effect on energy levels of D orbitals. It concentrates on how the electronic structure of the ligand affects the metal’s D orbitals, causing a decrease in the energy they generate.
Implications for practical use: spectrochemical series: Understanding the spectrochemical series can help to predict and explain the colors and absorption spectra seen in complexes of transition metals. The spectrochemical series has practical applications in fields like the study of materials, coordination, and bioinorganic chemistry. exact control of the optical and color properties is essential.
Nephelauxetic series: Nephelauxetics helps in the prediction of the stability of metal complexes as well as understanding the ligand field splitting. It is applicable to fields like catalysis medicinal chemistry, and biochemistry in which the stability and reactivity of the metal complexes are crucial.
Although all of Spectrochemical as well as the Nephelauxetic Series relate to ligand interactions upon transition metal complexes they focus on different areas and focus on different aspects. The spectrochemical series focuses primarily on aspects of electronic structure and the colors of complexes however, the nephelauxetics series is focused on the energy and reactivity of d orbitals as well as stability. Understanding the Spectrochemical as well as the Nephelauxetic Series provides a more complete understanding of the optical, electronic, and reactivity properties that metal compounds exhibit.
Importance of understanding the differences between Spectrochemical and Nephelauxetic Series
Understanding the differences between the Spectrochemical and Nephelauxetic Series:
Prediction of colors and absorption spectra: the spectrochemical series provides insights into how ligands influence the electronic properties of transition metal complexes, specifically their absorption spectra and colors. By understanding the factors affecting the spectrochemical series, scientists and chemists can predict and explain the observed colors of metal complexes.
Designing dyes and pigments: the spectrochemical series helps in the design and synthesis of dyes and pigments with specific colors. By selecting appropriate metal ions and ligands based on their positioning in the spectrochemical series, chemists can create compounds with desired absorption and reflection properties. This is important in industries such as textile manufacturing, cosmetics, and art.
Predicting stability and reactivity: the nephelauxetic series, on the other hand, focuses on the ligand’s effect on the energy of d orbitals in transition metal complexes. Understanding the nephelauxetic series allows scientists to predict the stability of metal complexes. This knowledge is valuable in fields such as catalysis, biochemistry, and medicinal chemistry.
Controlling properties and reactivity: the nephelauxetic series also plays a role in controlling the reactivity and catalytic properties of transition metal complexes. By choosing ligands with specific field strengths and understanding their impact on the energy of d orbitals, chemists can modulate the electronic structure and properties of metal complexes. This is crucial in fields such as homogeneous catalysis, where precise control over reactivity is essential.
Comprehensive understanding: while the spectrochemical series and nephelauxetic series focus on different aspects of transition metal complexes, understanding both provides a more comprehensive understanding of these systems. By considering both series, researchers can gain insights into the electronic, optical, and reactivity properties of metal complexes, leading to more accurate predictions and rational design strategies.
Understanding the differences between the Spectrochemical and Nephelauxetic Series series is crucial for predicting colors, designing dyes and pigments, assessing stability and reactivity, controlling properties and reactivity, and achieving a comprehensive understanding of transition metal complexes. This knowledge has practical applications in various scientific and industrial fields, contributing to advancements in materials science, chemistry, and related disciplines.
Conclusion
Understanding the differences between the Spectrochemical and Nephelauxetic Series is important for a comprehensive understanding of the effects of ligands on transition metal complexes.
The spectrochemical series focuses on the ligand’s influence on the electronic properties, absorption spectra, and colors of complexes. It considers factors such as ligand charge, size, and oxidation state. By understanding the spectrochemical series, scientists can predict and explain the observed colors of metal complexes, aiding in applications such as designing dyes and pigments with specific optical properties.
