Thymolphthalein and phenolphthalein are chemical compounds known as pH indicators used in laboratory settings. They both exhibit color changes in response to variations in the acidity or alkalinity of a solution.
Thymolphthalein changes from colorless to blue under alkaline conditions, making it useful in determining the endpoint in titrations involving strong bases. It transitions through various shades of yellow and blue as the pH level shifts.
Phenolphthalein, on the other hand, undergoes a color change from colorless to pink or red in alkaline solutions. It is commonly employed as an acid-base indicator in titrations involving strong acids and weak bases, where it shifts from clear to pink at a specific pH range.
Though both compounds are pH indicators, their distinct color changes in response to different pH levels make them suitable for specific types of chemical analyses and titrations in laboratory experiments.
What is Thymolphthalein (Thymol)?
Thymolphthalein, a type of phthalein dye, is used as an indicator of acid-base. The chemical formula for thymolphthalein (C28H30O4) is C28H30O4.
It is a color indicator that changes its hue when the pH of a reaction mixture changes:
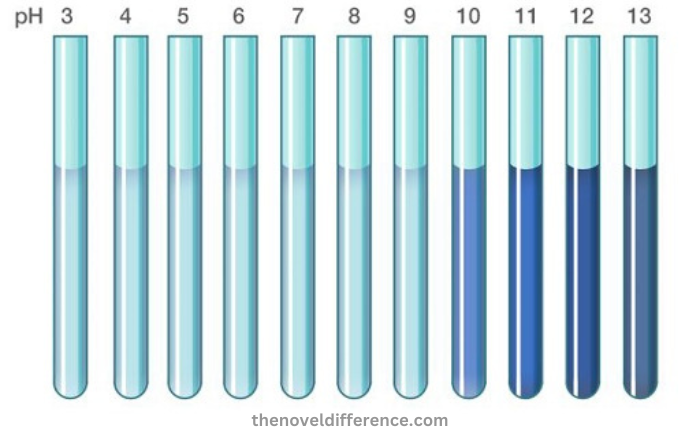
This indicator has a transition pH range between 9.3 and 10.5. Thymolphthalein appears blue at pH values higher than 10.5. It is colorless under pH 9.3. The molar extinction of thymolphthalein at 595 nm is 38 000 M 1cm 1.
Thymol and phthalic acid can be used to synthesize thymolphthalein. This reaction produces a white powder, which is the form of thymolphthalein that is commercially available. This substance decomposes at high temperatures. This substance can also be used as a laxative or to dissolve ink.
What is Phenolphthalein (Phenolthalein)?
Phenolphthalein, a pH indicator, is useful for acid-base titration. This is an indicator we use frequently in laboratory titration procedures.
This substance has the chemical formula C20H14O4:

This term can be written as “Hin”, “phph”, or “Hin”. The basic color of Phenolphthalein, however, is pink. This color change occurs in a pH range of 8.3 to 10.0.
The phenolphthalein is also water-soluble and dissolves easily in alcohols. So, they can be used in titrations. Phenolphthalein, a weak acid, can release protons into the solution.
It is colorless and nonionic. It is pink in color and is an ionic phenolphthalein. The equilibrium between the nonionic and ionic forms shifts towards the deprotonated form when we add basic to the reaction mixture containing phenolphthalein.
In order to synthesize phenolphthalein, we need two equivalents of phenol in acidic conditions. This reaction can also be catalyzed by a mixture consisting of zinc chloride, thionyl chloride, and other chemicals.
The difference between Thymolphthalein and phenolphthalein
Here are the main differences between thymolphthalein (phenolphthalein) and thymolphthalein:
Chemical Structure:
- The molecular form of thymolphthalein is C28H30O4, whereas the molecular form of phenolphthalein is C20H14O4. The differences in their molecular forms indicate variations in the chemical composition and structural characteristics of these compounds.
Physical Properties:
- Thymolphthalein looks like a white, pale yellow, or even a clear crystalline substance. Phenolphthalein is colorless.
- Thymolphthalein melts at around 130-132degC. Phenolphthalein melts at 258-263degC.
- Thymolphthalein dissolves in organic solvents such as ethanol and acetone but is only very slightly soluble when mixed with water. Unlike phenolphthalein, which is insoluble with water but soluble with organic solvents.
Acid-Base Indicator Properties:
- Thymolphthalein changes color from colorless to blue when it is in contact with a strong base. This makes it a good indicator of alkaline conditions.
- Phenolphthalein is colorless when used in an acidic solution. However, it turns pink or even red when mixed with a base. This occurs most often in pH values between 8.2 and 10.0.
Applications:
- Thymolphthalein, a common acid-base indicator used in laboratory experiments, is also found in the medical and pharmaceutical fields. It can be used to assess gastric acid secretion or for medical diagnostic tests.
- Phenolphthalein, a common acid-base indicator used in laboratory experiments and for titrations, is widely used. It was also used in certain pharmaceutical formulas as a laxative, but its use in this capacity has been discontinued by some regions because of safety concerns.
Safety Considerations:
- Thymolphthalein can cause irritation of the eyes and skin. It is important to use protective equipment and handle it properly. It has no significant environmental impact.
- Phenolphthalein can cause irritation of the skin and eyes. Exposure to phenolphthalein for a long time or repeatedly can have adverse effects on health. If released into the water, phenolphthalein may also have adverse effects on aquatic life.
While thymolphthalein (and phenolphthalein) are both acid-base indicators, their chemical structure, physical characteristics, and applications differ.
What are the benefits of thymolphthalein and phenolphthalein?
The use of phenolphthalein and thymolphthalein in different applications has many benefits:
Thymolphthalein benefits:
- Acid-base indicators: Thymolphthalein, a common acid-base marker in laboratory experiments. Its color changes from colorless blue to colorless in the presence a strong base, making it easy to detect and monitor alkaline conditions.
- Thymolphthalein can be used in medical diagnostic tests for determining the pH of body fluids. This is particularly useful when assessing acid secretion from the stomach. It helps diagnose conditions relating to acidity and gastric function.
Phenolphthalein benefits:
- Acid-base indicators: Phenolphthalein, a common acid-base marker used in laboratory experiments and for titrations. Its color changes from colorless into pink or red when a base is present, allowing for accurate endpoint detection of acid-base reactions.
- Phenolphthalein is used in pharmaceuticals to stimulate bowel movements. It is important to know that the use of phenolphthalein as a laxative was discontinued in certain regions because of safety concerns.
Thymolphthalein, as well as phenolphthalein, are important acid-base indicators. They allow for the identification and characterization in different environments of acidic or alkaline conditions.
They are used in medical diagnostics and laboratory experiments to understand and monitor chemical reactions and physiological processes. It is important to use these compounds in a responsible manner and according to safety guidelines.
What are the advantages and disadvantages of Thymolphthalein and phenolphthalein?
Although thymolphthalein has its benefits, it also has some drawbacks and considerations.
Thymolphthalein has some disadvantages:
- Thymolphthalein has a limited pH range. It changes color in a narrow range of pH, usually in the presence strong bases. It may not be suitable to detect or indicate acidity or pH values outside of its specific range.
- Environmental factors can affect thymolphthalein’s color. Temperature, light and impurities may influence its accuracy and reliability.
Phenolphthalein has some disadvantages:
- Like thymolphthalein: It has a limited pH range. It may not work to detect or indicate acidity levels beyond this range.
- Safety Concerns: Phenolphthalein is associated with health risks. This is especially true with repeated or prolonged exposure. Some regions have discontinued its use as a laxative due to fears about the potential carcinogenicity of phenolphthalein and other adverse effects.
- Phenolphthalein: Has an adverse impact on aquatic organisms when released into bodies of water. To minimize contamination of the environment, it is important to use proper disposal methods.
Consideration should be given to the potential environmental impact, safety precautions and limitations of the use of thymolphthalein or phenolphthalein. To ensure safe handling and application, it is important to adhere to safety guidelines and regulatory standards.
Safety Considerations
Thymolphthalein:
- Warnings and precautions about thymolphthalein: It can cause irritation to the eyes and skin. When working with this substance, it is important to use protective equipment such as safety goggles and gloves.
- Environmental Impact Thymolphthalein has no significant environmental impact. It should still be disposed of according to local regulations in order to minimize potential negative effects.
Phenolphthalein:
- Warnings and precautions regarding phenolphthalein: This compound is potentially hazardous. It can cause irritation of the eyes and skin, and repeated or prolonged exposure to it may have adverse effects on health. Handling phenolphthalein safely is essential. Wearing safety goggles and gloves are recommended.
- Phenolphthalein has a negative impact on aquatic organisms when released into bodies of water. To prevent contamination of the environment, it is important to use proper disposal methods.
When handling chemicals such as thymolphthalein or phenolphthalein it is important to refer to the safety data sheets and adhere to recommended safety practices.
Apps
Thymolphthalein:
- Thymolphthalein can be used in a variety of laboratory experiments, including titrations and acid-base experiments.
- Applications in medicine and pharmacology: It can be used to determine the pH of body fluids or assess gastric acid production.
Phenolphthalein:
- Phenolphthalein can be used in laboratory experiments to determine acid-base levels.
- Applications in medicine and pharmaceuticals: It’s used in medical tests as an indicator, such as acid-base balance assessments, and in some pharmaceutical formulations as a laxative. Some countries have stopped using phenolphthalein as a laxative due to safety concerns.
The conclusion of the article
Thymolphthalein is a white or pale yellow crystalline solid that undergoes a color change from colorless to blue in the presence of phenolphthalein. Thymolphthalein, a white or pale-yellow crystalline solid, changes color from colorless into blue when exposed to a strong base.
Phenolphthalein is a colorless, crystalline solid that turns pink or orange in alkaline conditions. Thymolphthalein is a different substance with a higher molecular mass and different solubility than phenolphthalein. Both are used in lab experiments as indicators of acid-base, and thymolphthalein is also used medically to assess gastric acid secretion.
Phenolphthalein was previously used as a laxative. The safety considerations are the potential irritation of the eyes and skin. Proper precautions must be taken when handling.
