Overview of ethylene dichloride and ethylidene Chloride
A brief overview of ethylene Dichloride (EDC): This hydrocarbon is produced primarily by ethane chlorination. Both organic and water-soluble solvents can be used. The optimal boiling temperature for this process is 57.2degC (135.5″F). EDC is produced primarily by the direct chlorination of ethylene. It is soluble in a variety of organic solvents, but not in water. EDC is used in the production process of vinyl chloride monomer (VCM), and is then further processed into polyvinylchloride (PVC), an important plastic material. It is also used as a solvent for different chemicals and as a fumigant for grain.
A brief overview of 1,1-dichloroethane (EDC): Ethylidene Chloride is a colorless, sweet-smelling liquid. C2H4Cl2 is the chemical formula for Ethylidene. Ethane chlorination is one of the primary ways of producing this hydrocarbon from petroleum; organic solvents, as well as water-soluble solvents, may be used, with an optimal boiling temperature being 57.2degC (135.5degF). Ethylidene can also be used to dissolve various resins like cellulose acetate. A brief overview of 1,1 dichloroethane: Ethylidene chloride is a clear, colorless liquid with a sweet smell.
Notably, although both ethylene dichloride and ethylidene (abbreviated EDC) represent different chemical compounds, their abbreviations can often be confused as being interchangeable. Ethylene dichloride is usually abbreviated to EDC for short. Ethylidene Chloride can also be abbreviated to EDC in order to avoid confusion.
Understanding the difference between EDC & ethylidene Chloride is important
There are several reasons why it is important to understand the difference between ethylene Dichloride (EDC), and Ethylidene Chloride (EDC).
- Safety & Handling: EDC & ethylidene Chloride have different chemical characteristics, including boiling points and solubility. It is important to know these differences in order to properly handle, store, and transport these chemicals. This will ensure that workers and the environment are safe.
- Industrial Application: EDC is used for different industrial applications. EDC is used primarily in the manufacture of vinyl chloride monmer (VCM), and then polyvinylchloride (PVC), whereas ethylidene chlorine is used as a solvent, and intermediate in other chemical production. Understanding these differences helps industries choose the right chemical for their manufacturing processes.
- Environmental impact: EDC or ethylidene Chloride can have different environmental impacts because of differences in their persistence, biodegradability and toxicity towards aquatic organisms. Understanding these differences can help in assessing potential environmental risks and implementing appropriate waste management and disposal techniques.
- Regulatory compliance: Regulations, guidelines, and rules regarding the use, handling, and release of EDC or ethylidene chloride can vary. It is important to be familiar with the regulatory requirements of each chemical in order to avoid legal and financial penalties and ensure compliance.
- Risk Assessment and Mitigation: Understanding the differences between EDC, and ethylidene chloride will allow for an accurate risk assessment. This includes evaluating the exposure scenarios, determining the appropriate personal protective gear (PPE), implementing controls, and developing emergency reaction plans tailored to each chemical’s specific properties.
Understanding the differences between EDC, ethylidene chloride (EC), and EDC chloride is essential to promoting safety and selecting an appropriate chemical for any given application. You’ll also gain greater insight into its environmental impacts, regulations pertaining to it, and potential risks related to chemicals.
Chemical Properties
Chemical properties describe the behavior and reactions of a substance on a molecule scale.
Here are a few of the most important chemical properties of ethylene dichloride, and ethylidene chloride:
Ethylene Dichloride (EDC):
- Chemical Formula: C2H4Cl2
- EDC: EDC is composed of two chloride atoms bonded to an ethylene (C2H4) central molecule.
- Physical state: EDC at room temperature is a colorless fluid.
- Boiling point: EDC’s boiling point is 83.5degC.
- Solubility: EDC has limited solubility when dissolved in water.
- Reactivity: EDC undergoes a variety of chemical reactions. Due to its chlorine atoms, it can be involved in halogenation processes, including substitution and addition.
Ethylidene Chloride (EDC):
- Chemical Formula: C2H4Cl2
- Molecular structure: The ethylidene-chloride (CH3CHCl), consists of a central ethylidene molecule with two chlorine atoms.
- Physical state: Ethylidene Chloride is a colorless liquid when at room temperature.
- Boiling point: Ethylidene Chloride has a boiling point of approximately 57.2degC (135.5degF).
- Solubility: Ethylidene Chloride is soluble only in organic solvents. It has limited solubility when it comes to water.
- Reactivity: Ethylidene Chloride is also reactive, and can undergo chemical reactions similar to EDC. It can be used in halogenation and other substitution or addition reactions.
EDC and Ethylidene Chloride both have similar chemical formulas, and they also exhibit similar chemical reactions due to the chlorine atoms. Their specific physical properties such as boiling point may vary, which could impact their application and behavior in various chemical processes.
What Is Ethylene Dichloride (EDC)?
Ethylene Dichloride (EDC), commonly referred to as 1,2-dichloroethane, is a chemical compound with the molecular formula C2H4Cl2. It’s a colorless liquid with an unpleasant chloroform-like scent.
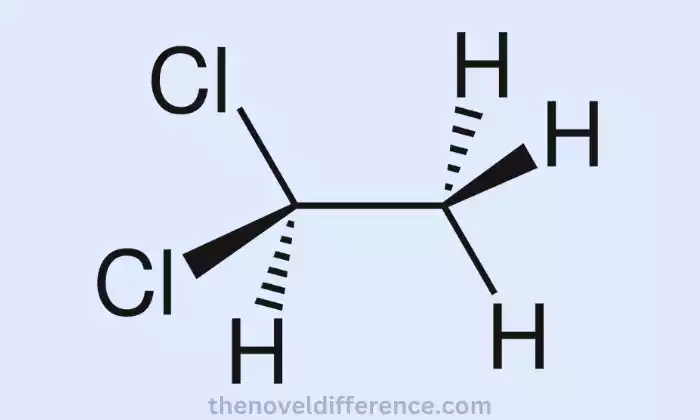
EDC is used as an intermediate in the production of vinyl chloride monomer (VCM), which in turn is processed to form polyvinyl chloride (PVC), an extremely versatile plastic material.
EDC is made by directly chlorinating ethylene (C2H4) with the aid of a catalyst and is highly reactive due to the presence of two chlorine atoms attached to its structure. As a result, EDC has an exceptionally high boiling point at around 83.5degC (182.3degF). Furthermore, its solubility in organic solvents but only minor solubility in water makes EDC readily accessible as an organic compound.
PVC feedstocks have long been utilized by industry for the production of PVC plastic, an extremely durable plastic that stands out for its versatility, resistance to chemicals, and weatherproof properties.
EDC can also be used as a solvent for various materials, including fats, oils, waxes, and synthetic materials. Furthermore, it has also been utilized as a grain storage fumigant to combat pest infestation and minimize microbial growth.
Note that EDC must be handled and used according to relevant safety measures and regulations in order to reduce potential health and environmental risks.
What Is Ethylidene Chloride?
Commonly referred to as ethylidene chloride, chloroethane is actually known by another name – ethyl chloride.
Chloroethane (C2H5Cl), with the chemical formula C2H5Cl, is a colorless gas or liquid at room temperature depending on pressure. It possesses a pleasant sweet, ether-like fragrance and is highly flammable.
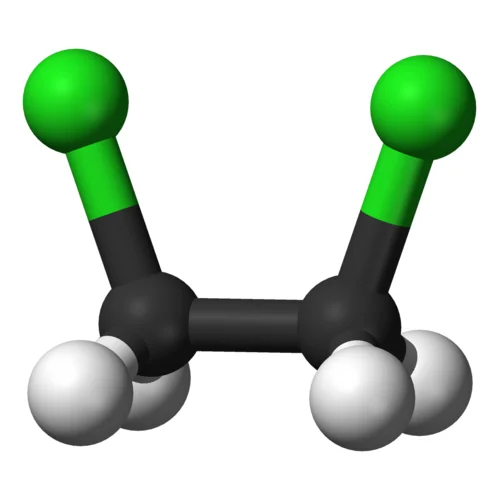
Chloroethane is widely utilized as a solvent across numerous industries. It can be found in pharmaceutical production, intermediate chemical manufacturing processes and refrigeration operations, refrigerant applications, and aerosol propellant production. Chloroethane was even once an essential part of lead-based gasoline.
Due to potential health and environmental risks associated with chloroethane use, its applications are relatively limited compared to other chlorinated compounds. Production and usage have declined over recent years as concerns about its toxicity and ozone-depleting potential have diminished its production and usage.
Please accept my apology for any confusion caused, and I hope this clears up information regarding ethylidene chloride, more commonly referred to as chloroethane or ethyl chloride.
What is the difference between Ethylene dichloride and Ethylidene chloride?
Chemical structures and properties are the main differences between ethylene dichloride and ethylidene chloride. Here are some key differences.
- Chemical structure:
-
- Ethylene Dichloride: EDC is a chemical compound with the formula C2H4Cl2. It is composed of two atoms of chlorine (Cl), attached to a central ethylene molecule (C2H4).
- Ethylidene Chloride: Ethylidene is not a specific compound. If you are referring to ethyl or chloroethane, the chemical formula for this compound is C2H5Cl. It has an ethyl (C2H5) group attached to a chlor (Cl).
- Physical state:
-
- EDC: At room temperature, ethylene dichloride appears as a colorless liquid.
- Ethylidene chloride (Chloroethane): Chloroethane is a liquid or gas depending on the temperature and pressure. It can be a liquid or a gas depending on the conditions.
- Synthesis:
-
- EDC: The primary method of producing ethylene dichloride (C2H4) is by direct chlorination.
- Ethylidene chloride (Chloroethane): The reaction between ethylene and hydrogen chloride in the presence of a catalyst produces chloroethane.
- Uses:
-
- EDC: Ethylene Dichloride is used in the production process of polyvinyl chloride (PVC) and vinyl chloride monomer. It can also be used as a grain fumigant and solvent.
- Ethylidene chloride (Chloroethane): Chloroethane is used as a refrigerant and aerosol propellant. It can also be used as a local anesthetic.
Chemical structures, physical states, and synthesis methods are the main differences between ethylene Dichloride (EDC), and ethylidene Chloride (chloroethane).
EDC is 1,2-dichloroethane, (C2H4Cl2) while ethylidene chloride (C2H5Cl) usually refers to a different compound that has its own unique properties and applications.
Uses and Applications
The following are the uses and applications for Ethylene Dichloride and Ethylidene Chloride, also known as chloroethane.
Ethylene Dichloride (EDC):
- Production Vinyl Chloride Monomer (VCM): EDC’s primary use is as an intermediary in the production of vinyl chloride monomer. VCM can be further processed into polyvinylchloride (PVC), which is a versatile material that’s used for construction, pipes, and electrical cables.
- Solvent: EDC can be used to dissolve a variety of substances including oils, fats, waxes, and resins.
- Fumigant For Grain: Due to its pesticidal qualities, EDC is used to fumigate stored grain in order to control pests as well as inhibit microbial development.
Ethylidene Chloride (Chloroethane):
- Solvent: You can use chloroethane to dissolve organic compounds. It can be used in the production of adhesives, coatings, and cleaning agents.
- Aerosol Propeller: Chloroethane can be used as a propellant for aerosol products such as sprays or foams.
- Refrigerant: Due to its low boiling temperature and heat-absorbing qualities, chloroethane is used in some applications as a refrigeration agent.
- Chemical Intermediary: The chloroethane is an intermediate used to synthesize various chemicals. This includes vinylidene, which can be used to produce polymers that have high gas barrier properties.
While both EDC and chloroethane are used as industrial solvents, the specific industries and uses may vary. EDC is primarily used as a VCM intermediate, whereas chloroethane has broader uses as a refrigerant and propellant.
Toxicity of Chemicals and Their Health Effects
Below are the toxicities and health effects associated with Ethylene dichloride (EDC), Ethylidene chloride (Chloroethane), and Ethylidene chloride (Chloroethane).
Ethylene Dichloride (EDC):
- Inhalation: Inhaling EDC vapors may cause irritation of the respiratory tract, such as coughing, breathing difficulties, and chest pain. Long-term or high-level exposure can cause lung damage, chemical pneumonia, or pulmonary swelling.
- Eye and Skin contact: Direct skin exposure to EDC can cause irritation and dermatitis. Contact with the eyes can cause irritation, dermatitis, and even corneal injury.
- Ingestion: EDC can cause nausea, vomiting, and abdominal pain. Chemical burns may cause serious damage to the digestive tract in some cases.
- Chronic exposure: EDC exposure over a prolonged period or repeated exposure has been associated with liver and renal damage, as well as effects on the nervous system. It has been classified as a possible human carcinogen by some regulatory authorities, but there is limited evidence. Further research is required.
Ethylidene Chloride (Chloroethane):
- Inhalation exposure: Inhalation can cause respiratory irritation including coughing, sore mouth, and difficulty in breathing. Dizziness, headaches, and even loss of consciousness can occur at high levels of exposure.
- Contact with Skin and Eyes: Direct skin contact may cause irritation, erythema, and dermatitis. Contact with the eyes can cause irritation, redness, and even corneal damage.
- Ingestion: Chloroethane ingestion can cause nausea, vomiting, and abdominal pain. Ingestion is rare, as the chemical is used primarily in industrial applications.
- Chronic exposure: Long-term or repeated exposures to chloroethane have been linked with kidney and liver damage. It can also affect the nervous system, leading to symptoms like dizziness, confusion, and coordination problems.
Inhalation is a primary exposure route for both EDC (and chloroethane). For minimizing exposure risks and ensuring worker safety, it is important to use personal protective equipment and adhere to safety guidelines.
For detailed information about the toxicity of these chemicals and their safety, it is best to consult safety data sheets or guidelines from reliable sources.
Environmental Impact
Ethylene Dichloride and Ethylidene Chloride, also known as Chloroethane (EDC), have the following environmental impacts:
Ethylene Dichloride (EDC):
- Persistence: EDC can be persistent for a very long time in the environment. This characteristic can cause its accumulation in soils, water and organisms.
- Contamination of Water: Unsafe disposal or accidental releases can contaminate groundwater and surface waters. EDC isn’t highly soluble in the water but its persistence can be a threat to aquatic organisms.
- EDC is toxic: To aquatic organisms. EDC can be toxic to aquatic organisms. EDC in high concentrations can negatively affect aquatic organisms, including fish, invertebrates, and other species. This can lead to decreased reproduction, impaired growth and even death.
- EDC is classified as a VOC (volatile organic compound): Which is a chemical that can contribute to ozone and ground-level air pollution. VOCs can also have an indirect impact on the environment and human health because they are involved in smog production and chemical reactions that produce harmful byproducts.
Ethylidene Chloride (Chloroethane):
- Volatility: Chloroethane, a volatile compound is easily evaporated into the air. Volatile compounds are responsible for air pollution, the formation of ozone at ground level, and other harmful effects to human health.
- Contamination of Soil and Water: Improper disposal or accidental release of chloroethane may lead to contamination of soil and water sources. As this compound does not dissolve easily in water, it may pollute both surface soil and groundwater sources contaminating both of them with hazardous contaminants.
- Ecotoxicity: Chloroethane is toxic to aquatic organisms, and it can also harm terrestrial organisms when they come in contact with soil contaminated by chloroethane. Chloroethane in high concentrations can be toxic to aquatic organisms, such as fish and other invertebrates. This can affect their survival, reproduction and ecosystem health.
In order to minimize the environmental impacts associated with EDC or chloroethane, proper handling, storage and disposal practices must be observed. By adhering to regulatory requirements and choosing environmentally-friendly alternatives you can reduce their negative effect.
Regulations and safety measures
To protect the public’s health, ensure workplace safety and minimize environmental impact, regulations and safety measures were implemented. Here are a few examples:
- Occupational Safety and health Administration Standards (OSHA): OSHA enforces workplace safety laws throughout the United States. These standards apply to the handling of hazardous chemical substances in industrial settings. This includes EDC and Chloroethane. OSHA mandates the use of personal protective equipment (PPE), proper ventilation systems, and employee training to minimize exposure risks.
- Environmental Protection Agency Regulations: The Environmental Protection Agency establishes guidelines and regulations to manage the use, storage, transport and disposal of hazardous chemicals such as EDC or Chloroethan
e. These are intended to protect the environment by reducing air pollution levels and safeguarding water sources; in order to comply with them you must obtain permits, implement spill prevention/control methods and follow disposal guidelines. - Globally Harmonized System of Classification and Labelling of Chemicals: GHS is a standard system for identifying, classifying and labeling hazardous chemicals. EDC and Chloroethane containers should have clear labels with hazard information, such as pictograms and signal words.
- Guidelines for Storage and Handling: These guidelines provide specific instructions on how to store and handle chloroethane and EDC safely. These guidelines cover factors like the use of proper containers, compatibility issues with other chemicals and ventilation requirements. They also address spills and leaks and emergency procedures.
- Waste Disposal Regulations: Proper waste disposal of EDC, chloroethane and other chemicals is essential to avoid environmental contamination. The regulatory agencies give guidelines on the proper disposal methods such as recycling or treatment.
Individuals and organizations should stay up to date with the latest regulations and guidelines that apply in their region. Employers must train their employees in safe handling techniques, emergency procedures and personal protective equipment.
The strict adherence to the regulations and safety measures help protect human health, assure workplace safety and minimize the impact on the environment associated with the handling and use of EDC.
Conclusion
Understanding the differences between Ethylene Dichloride and Ethylidene Chloride can prevent confusion and give accurate answers when discussing properties, uses and toxicity of these chemicals, as well as their environmental impact and safety measures.
EDC, or 1,2-dichloroethane, is used as a solvent and an intermediate for the production of vinyl chloride monomer. Chloroethane can be used as a propellant as well as as a refrigerant.
Both EDC and chloroethane have associated health risks, including respiratory irritation, skin and eye irritation, and potential long-term effects on various organs. They also pose environmental concerns due to their persistence, potential water contamination, toxicity to aquatic life, and contribution to air pollution.
Regulations and safety measures set by organizations such as OSHA and the EPA play an essential part in mitigating risks associated with chemical handling, storage, transport, and disposal.
To protect human health, to ensure workplace safety and to reduce environmental impact, it is essential that you comply with all regulations, including those relating labeling, PPE use, ventilation systems and waste management.
