Photochromic Materials, think of these like the chameleons of the material world. When they soak up UV rays from the sun, they get darker. So, your eyeglasses that go from clear to shaded in the sunlight? That’s photochromic magic at work!
Thermochromic Materials are like mood rings for materials. They change color when they feel hot or cold. Imagine a cup that turns red when you pour a hot beverage into it and then goes back to its original color when it cools down.
So, photochromic materials react to UV rays, while thermochromic materials respond to temperature changes. Both of these terms are handy in scientific and everyday situations, especially when it comes to things like eyewear and cool color-changing products.
What are Photochromic Materials?
Photochromic materials are substances that have the remarkable property of changing color in response to exposure to ultraviolet (UV) radiation, typically from sunlight or artificial UV sources.
These materials undergo a reversible chemical or physical change when they absorb UV light, causing them to darken or change color. When the UV exposure diminishes or the source of UV radiation is removed, the photochromic material gradually reverts to its original state, often returning to its initial color.
Key characteristics of photochromic materials include:
- UV-Induced Color Change: When photochromic materials absorb UV radiation, they transition to a darker or different color state. This property is commonly used in lenses, eyeglasses, and sunglasses.
- Reversibility: The color change in photochromic materials is reversible, meaning that they can return to their original state when no longer exposed to UV light. This reversibility allows them to adapt to changing light conditions.
- Applications: Photochromic materials are widely used in eyewear, especially in transition lenses, which automatically adjust their darkness based on UV exposure. This feature provides both UV protection and visual comfort in varying light conditions.
- Advantages: Photochromic materials offer convenience and eye protection by reducing the need to switch between regular and tinted glasses when transitioning between indoor and outdoor environments.
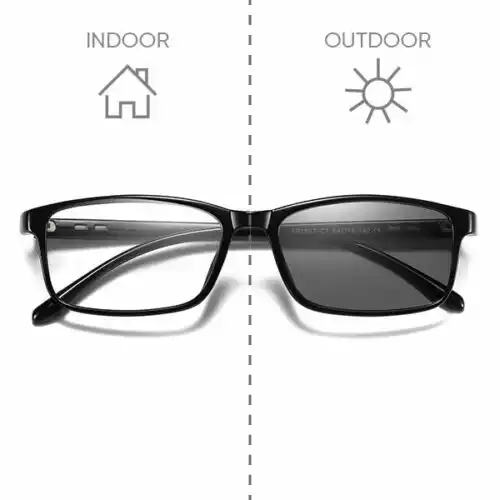
These materials are based on complex chemical reactions involving molecules called photochromic dyes. When exposed to UV light, these dyes undergo structural changes that result in a shift in their absorption spectrum, leading to the observed color change. Photochromic materials are valuable in various optical and photographic applications, as well as in products designed to enhance eye comfort and protection from UV radiation.
Is a photochromic lens good for the eyes?
Photochromic lenses, often referred to as transition lenses, are designed to automatically adapt to changing light conditions. They darken in response to UV radiation (such as sunlight) and become lighter when UV exposure diminishes, such as when indoors.
Whether photochromic lenses are good for your eyes depends on various factors and individual preferences:
Advantages:
- UV Protection: Photochromic lenses provide valuable protection against harmful ultraviolet (UV) radiation from the sun. Prolonged exposure to UV light can contribute to eye problems, such as cataracts and photokeratitis. Photochromic lenses help shield your eyes from these harmful rays.
- Convenience: These lenses eliminate the need for carrying and switching between multiple pairs of eyeglasses (e.g., prescription glasses and sunglasses) when transitioning between indoor and outdoor environments. They adapt to different light conditions automatically.
- Visual Comfort: Photochromic lenses reduce glare and improve visual comfort in varying lighting conditions, enhancing overall vision.
- Eye Fatigue Reduction: By optimizing light levels, photochromic lenses can help reduce eye strain and fatigue when moving between indoor and outdoor spaces.
Considerations:
- Transition Speed: The time it takes for photochromic lenses to fully adapt to changing light conditions can vary. Some people may find that the transition speed is not instantaneous, which can be a concern when moving from bright sunlight to indoor lighting.
- Intensity of Darkening: The extent to which photochromic lenses darken may vary between different brands and types. Some may not darken as much as dedicated sunglasses.
- Cold Weather: Photochromic lenses may not respond as effectively in cold weather, as the activation of the color change is often dependent on temperature and UV exposure.
- Impact on Driving: Photochromic lenses may not darken as effectively inside a car because the windshield typically blocks a significant portion of UV radiation. As a result, they might not provide adequate sun protection for driving.
- Individual Preferences: Some people prefer to have separate prescription sunglasses for outdoor use, as they can offer more consistent darkness and specific UV protection.
Photochromic lenses are generally good for your eyes because they offer UV protection, convenience, and visual comfort. However, the effectiveness and overall suitability of these lenses depend on individual needs and preferences, as well as specific environmental conditions. It’s important to discuss your eyewear choices with an optometrist or eye care professional to ensure they meet your vision and eye health requirements.
What are Thermochromic Materials?
Thermochromic materials are those that change color due to temperature variations. When heated or cooled, these materials undergo reversible color transitions, providing a visual indicator of temperature variations. They are often used in a wide range of applications to monitor or display temperature changes in a practical and engaging way.
Key characteristics of thermochromic materials include:
- Temperature-Induced Color Change: Thermochromic materials alter their color as a result of temperature fluctuations. When heated, they can transition to a different color state, and when cooled, they can revert to their original color.
- Reversibility: The color change in thermochromic materials is reversible, meaning that they can shift back to their initial color when the temperature changes again.
- Applications: These materials are employed in various consumer products, such as color-changing coffee mugs, baby bottles with temperature indicators, and thermometers. They also find applications in printing, textiles, and security features on documents.
- Practical Uses: Thermochromic materials are valuable in applications where a visual indication of temperature is beneficial, providing safety and convenience. For example, a color-changing baby bottle can help parents ensure the milk or formula is at an appropriate temperature for feeding.
- Microencapsulation: Many thermochromic materials consist of microcapsules that contain a mixture of dyes, which change color at specific temperature thresholds. These microcapsules are embedded in various products to enable the color-changing effect.

Thermochromic materials operate based on the principle that temperature affects the arrangement of molecules and dyes within them, leading to alterations in the absorption and reflection of light, resulting in visible color changes. These materials add an element of interactivity and functionality to a wide range of everyday objects and products.
What is the purpose of thermochromic?
The purpose of thermochromic materials is to provide a visual and reversible indication of temperature changes. These materials change color in response to alterations in temperature, and they have several practical applications:
- Temperature Monitoring: Thermochromic materials are used in various applications to monitor temperature changes visually. For example:
- In baby bottles: Thermochromic indicators on baby bottles change color to signal the temperature of the liquid, ensuring that it is at a safe and comfortable level for feeding.
- In cookware: Some thermochromic materials are applied to cookware to indicate when it has reached the desired cooking temperature.
- Safety Indicators: These materials are used to enhance safety by providing a visual indication of potential hazards related to temperature. For instance:
- Thermal labels: Thermochromic labels can be used in the transportation and storage of temperature-sensitive products, such as perishable foods and pharmaceuticals, to indicate if the items have been exposed to temperatures outside the recommended range.
- In automotive applications: Thermochromic paint can change color to warn of overheating or potential mechanical issues in engines.
- Consumer Products: Thermochromic materials are commonly found in consumer products to enhance user experience and engagement:
- In novelty items: Color-changing mugs and clothing are popular examples of thermochromic materials used for entertainment and aesthetic purposes.
- In promotional materials: Some companies use thermochromic ink for creative marketing campaigns or interactive packaging.
- Educational and Artistic Uses: Thermochromic materials have educational value and are used in art projects to demonstrate scientific principles related to temperature and color change.
- Quality Control: In industrial applications, thermochromic materials can be used to verify and control temperature-sensitive processes, such as heat sealing or heat treatment.
Thermochromic materials are versatile and serve a range of purposes, from practical applications like temperature monitoring and safety indicators to more creative and artistic uses in consumer products and educational settings. They offer an engaging way to visualize temperature changes and can enhance safety, convenience, and aesthetics in various contexts.
Key comparison chart
There’s a key comparison chart summarizing the main differences between photochromic and thermochromic materials:
| Characteristic | Photochromic Materials | Thermochromic Materials |
|---|---|---|
| Trigger for Color Change | UV radiation (sunlight or artificial UV sources) | Temperature variations (heating or cooling) |
| Reversibility | Reversible: Return to original state indoors | Reversible: Change back to original color with temperature changes |
| UV Protection | Provides protection against harmful UV radiation | Not designed for UV protection |
| Transition Speed | Speed of darkening/lightening may vary, not instant | Color change occurs relatively quickly with temperature shifts |
| Applications | Commonly used in eyewear (transition lenses) and optical products | Found in items like color-changing mugs, baby bottles, cookware, and clothing |
| Visual Comfort | Reduces glare and enhances comfort in changing lighting conditions | Offers visual feedback about temperature variations |
| Consistency | Darkening depends on UV intensity; may not be as effective inside a car | Color changes based on temperature variations |
| Effectiveness in Cold Weather | Works effectively in cold weather conditions | May not respond as well in very cold environments |
| Individual Preferences | A matter of personal preference and practicality; some people prefer separate sunglasses | Valued for their novelty and practicality in specific applications |
| Safety Indicators | Primarily used for UV protection and visual comfort | Used for temperature monitoring, safety warnings, and quality control |
This chart highlights the key differences between photochromic and thermochromic materials in terms of their triggers, applications, and advantages, which cater to various needs and preferences.
Importance of photochromic and thermochromic
Photochromic and thermochromic materials are pretty nifty and they have some really cool uses. Let’s break it down:
Why Photochromic Materials Are Important:
- UV Protection: These materials are like sunglasses for your eyes. They shield you from harmful UV radiation, reducing the risk of eye conditions like cataracts.
- Convenience: Ever heard of transition lenses? They automatically adjust from clear indoors to shaded in sunlight. No more switching between regular and sunnies!
- Visual Comfort: Glare is annoying. Photochromic lenses reduce it and make your vision more comfortable when you’re moving between different lighting conditions.
- Less Eye Strain: Your eyes get tired if they’re constantly adjusting to changing light. Photochromic materials help reduce eye strain, which is great for folks who spend lots of time outdoors.
- Better Eye Health: UV-blocking glasses in different settings protect your eyes from long-term UV damage, keeping your peepers healthy.
Why Thermochromic Materials Matter:
- Temperature Tracking: These materials are like temperature detectives. They change color to show you when things get hot or cold, which can be super useful.
- Safety Signs: In machines or food storage, thermochromic materials act as safety alarms, warning of overheating or temperature issues.
- Cool Consumer Stuff: Color-changing clothes, mugs, and fun products? That’s the work of thermochromic materials, adding a dash of fun to your life.
- Quality Control: In industries, these materials ensure that temperature-sensitive processes are spot on, maintaining product quality and safety.
- Learning and Art: They’re not just practical; they’re educational and artistic too. You can use them to teach science or create cool art projects.
- Energy Savers: Thermochromic windows and coatings help control indoor temperatures, which can make buildings more energy-efficient and save you money.
Both photochromic and thermochromic materials are pretty important. Photochromic materials keep your eyes safe and comfy, while thermochromic materials play a role in safety, fun products, quality control, and even energy efficiency. They’re like magical chameleons that adapt to their surroundings and make life easier and more interesting.
Limitations of photochromic and thermochromic
Photochromic and thermochromic materials offer exciting benefits and applications, but they also come with certain limitations. Let’s explore the drawbacks of each:
Limitations of Photochromic Materials:
- Transition Speed: Photochromic lenses may not transition instantaneously, which can be a concern when moving from bright sunlight to indoor lighting. Some individuals prefer faster changes.
- Effectiveness Inside Vehicles: Photochromic materials may not darken as effectively when inside a car because windshields often block a significant portion of UV radiation. This can limit their sun protection for drivers.
- Limited Darkness: The extent to which photochromic lenses darken can vary between brands and types. Some may not become as dark as dedicated sunglasses, which can be a drawback in very bright conditions.
- Cold Weather Performance: Photochromic materials work well in cold conditions, but their performance might be slightly affected by extreme cold temperatures.
- Transition Speed Variation: Transition speed can vary depending on the specific photochromic material and the surrounding UV conditions, resulting in different experiences for wearers.
Limitations of Thermochromic Materials:
- Temperature Sensitivity: The color change in thermochromic materials is temperature-dependent, which means it might not provide consistent color changes in certain environments or when exposed to rapid temperature fluctuations.
- Durability: The durability of thermochromic materials can be a concern in some applications. Frequent color changes, exposure to harsh conditions, or repeated washing (in the case of clothing) can wear out the thermochromic properties over time.
- Limited Temperature Range: Thermochromic materials operate within specific temperature ranges, and their color transitions are triggered only within these ranges. They may not be suitable for extreme temperature monitoring.
- Reversibility Concerns: While they are generally reversible, the effectiveness of thermochromic materials in returning to their original color may vary based on the material and its exposure to temperature changes.
- Environmental Sensitivity: Thermochromic materials may be influenced by ambient temperature and might not function as expected in extremely hot or cold conditions.
While photochromic and thermochromic materials offer exciting features, they have their limitations. It’s essential to consider these limitations when choosing and using products that incorporate these materials to ensure they meet your specific needs and environmental conditions.
Conclusion
Both photochromic and thermochromic materials have their advantages and limitations. Photochromic materials are valuable for UV protection and visual comfort but may have slower transitions and limitations inside vehicles.
Thermochromic materials offer temperature monitoring and safety indicators but are sensitive to temperature variations and have durability concerns. Understanding these limitations is essential for making informed choices in various applications.
