Beryllium and lithium are two components with particular properties that set them separated from each other. We will investigate the contrasts between beryllium and lithium, investigating their nuclear structures, physical and chemical properties, and their different applications. By understanding the disparities between these two components, we can pick up a more profound understanding of their centrality and commitment to distinctive areas of science and innovation.
Importance and applications of Beryllium and Lithium
Aerospace and defense industry: Beryllium is profoundly esteemed in aviation and defense applications due to its remarkable strength-to-weight proportion, warm soundness, and electrical conductivity. It is used in aircraft and missile structures, satellite components, and high-performance alloys for jet engines.
Nuclear industry: Beryllium is utilized in nuclear reactors as a neutron reflector and moderator due to its ability to effectively reflect and moderate neutrons. It makes a difference in controlling atomic parting responses and improving the productivity and security of nuclear control plants.
Electronics and telecommunications: Beryllium’s electrical and thermal conductivity properties make it valuable in electronic components such as connectors, switches, and heat sinks. It is also used in telecommunications equipment and high-frequency devices.
Batteries and energy storage: Lithium-ion batteries have revolutionized convenient gadgets and electric vehicles. Lithium’s tall vitality thickness and fabulous electrochemical properties make it a perfect choice for rechargeable batteries, giving long-lasting control and speedier charging times.
Pharmaceuticals and medication: Lithium compounds, especially lithium carbonate, are utilized as disposition stabilizers within the treatment of bipolar clutter. They help regulate neurotransmitter activity in the brain, reducing mood swings and manic episodes.
Aerospace and automotive industries: Lithium is utilized in lightweight alloys for aerospace and automotive applications, contributing to fuel efficiency and improved performance. It is additionally utilized within the fabricating of flying machine parts, such as fuselage components and auxiliary materials.
Both Beryllium and Lithium play crucial roles in various industries and technologies, but their applications differ significantly. Beryllium finds its niche in high-performance applications where its exceptional strength, lightweight, and thermal properties are advantageous, such as aerospace, defense, and nuclear industries.
Lithium has picked up noticeable quality basically within the field of vitality capacity, especially with the far-reaching appropriation of lithium-ion batteries, which control a run of gadgets from smartphones to electric vehicles. Lithium has significant medical applications in treating bipolar disorder, while its lightweight alloys contribute to advancements in aerospace and automotive technologies. Understanding the unique properties and applications of these elements is essential for harnessing their benefits while ensuring safety and sustainability.
Definition of Beryllium and Lithium
Beryllium: Beryllium is a chemical element with the symbol Be and atomic number 4. It is a lightweight, silvery-white metal that belongs to the alkaline earth metal group of elements in the periodic table. Beryllium is known for its uncommon strength-to-weight proportion, tall softening point, and great warm conductivity. It is one of the lightest and stiffest metals, making it profitable for different mechanical applications. Beryllium incorporates a moo thickness, is non-magnetic, and stands up to erosion, which contributes to its appropriateness in basic situations such as aviation, defense, and atomic businesses. In any case, it is imperative to note that beryllium is exceedingly poisonous and postures wellbeing dangers when breathed in as fine particles or exhaust.
Lithium: Lithium is a chemical element with the symbol Li and atomic number 3. It is the lightest metal and the least dense solid element. Lithium has a place in the soluble base metal bunch within the periodic table and could be a delicate, silvery-white metal. It is highly reactive and flammable, reacting vigorously with water and oxygen. Lithium is Eminent for Its Amazing Electrochemical Properties, Making It a Crucial Component Within the Generation of Rechargeable Lithium-Ion Batteries. These batteries are broadly utilized in versatile gadgets, electric vehicles, and renewable vitality capacity frameworks due to their tall vitality thickness and long-lasting execution.
Lithium is additionally utilized in different other applications, counting pharmaceuticals for treating bipolar clutter, lightweight combinations in aviation and car businesses, and as a coolant in atomic reactors.
Atomic and Chemical Properties
Beryllium:
• Atomic number: 4
• Atomic symbol: Be
• Electron configuration: 1s^2 2s^2
• Atomic mass: 9.0121831 atomic mass units
• Beryllium is an alkaline earth metal and belongs to Group 2 of the periodic table.
• It has a relatively small atomic radius and high ionization energy.
• Beryllium has a low electronegativity and tends to form covalent bonds with other elements.
• It has a valence of +2 and typically loses two electrons to achieve a stable configuration.
• Beryllium has a relatively low melting point of 1,287 degrees Celsius and a boiling point of 2,471 degrees Celsius.
• It exhibits a metallic luster and is a good conductor of electricity and heat.
• Beryllium compounds are often highly toxic, particularly when inhaled as dust or fumes.
Lithium:
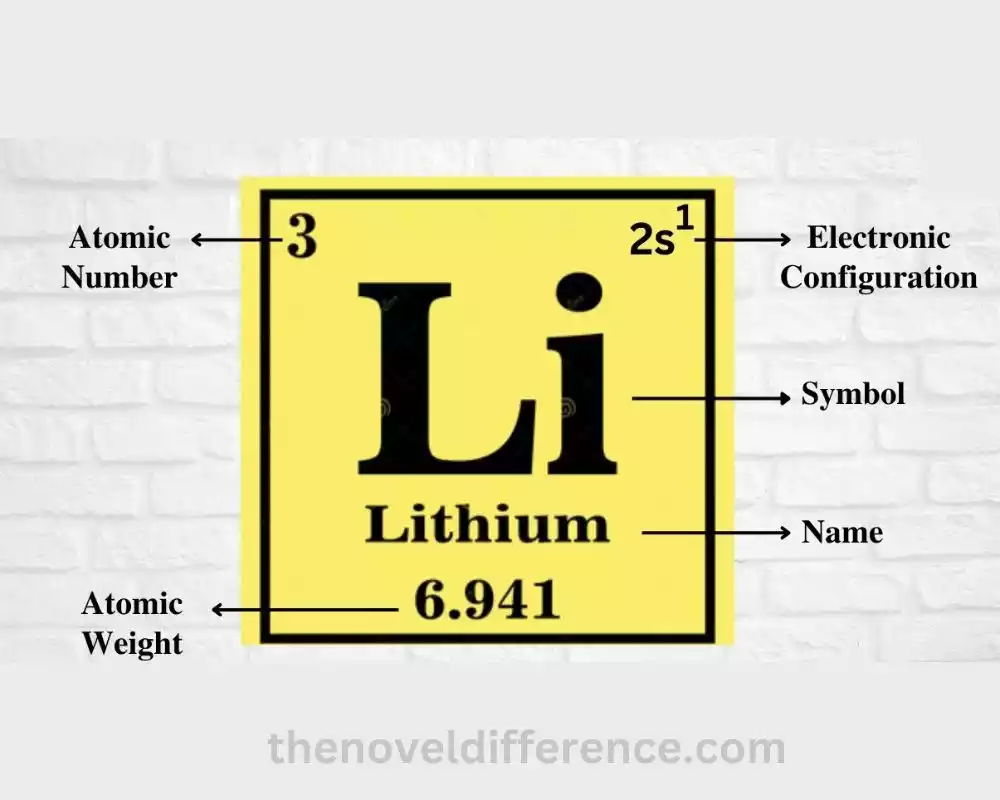
• Atomic number: 3
• Atomic symbol: Li
• Electron configuration: 1s^2 2s^1
• Atomic mass: 6.941 atomic mass units
• Lithium is an alkali metal and belongs to Group 1 of the periodic table.
• It has a larger atomic radius compared to beryllium.
• Lithium has a low density and is the lightest metal.
• It has a low electronegativity and tends to form ionic bonds with other elements.
• Lithium has a valence of +1 and easily loses one electron to achieve a stable configuration.
• It has a relatively low melting point of 180.5 degrees Celsius and a boiling point of 1,342 degrees Celsius.
• Lithium is a good conductor of electricity and heat.
• Lithium compounds, such as lithium carbonate, exhibit important pharmaceutical properties and are used as mood stabilizers in the treatment of bipolar disorder.
Physical Properties
Beryllium:
• Appearance: Beryllium is a silvery-white, lustrous metal.
• Color: It has a grayish-white color.
• Texture: Beryllium has a smooth and solid texture.
• Melting Point: The melting point of beryllium is 1,287 degrees Celsius (2,349 degrees Fahrenheit).
• Boiling Point: Beryllium has a high boiling point of 2,471 degrees Celsius (4,480 degrees Fahrenheit).
• Density: Beryllium is a lightweight metal with a density of 1.85 grams per cubic centimeter.
• Specific Gravity: The specific gravity of beryllium is 1.85, which means it is about 1.85 times heavier than water.
Lithium:
• Appearance: Lithium is a soft, silvery-white metal.
• Color: It has a silver color when freshly cut, but it quickly tarnishes to a grayish color upon exposure to air.
• Texture: Lithium is a soft and malleable metal that can be easily cut with a knife.
• Melting Point: The melting point of lithium is 180.5 degrees Celsius (356.9 degrees Fahrenheit).
• Boiling Point: Lithium has a relatively low boiling point of 1,342 degrees Celsius (2,448 degrees Fahrenheit).
• Density: Lithium is the least dense solid element, with a density of 0.534 grams per cubic centimeter.
• Specific Gravity: The specific gravity of lithium is 0.534, which means it is about 0.534 times heavier than water.
It’s vital to note that the physical properties specified over are normal values and may change somewhat depending on the particular conditions and isotopic composition of the components.
Physical properties of Beryllium
Physical properties of Beryllium include:
1. Appearance: Beryllium is a lightweight, silvery-white metal.
2. Color: It has a grayish-white color.
3. Texture: Beryllium has a smooth and solid texture.
4. Melting Point: The melting point of Beryllium is relatively high at 1,287 degrees Celsius (2,349 degrees Fahrenheit).
5. Boiling Point: Beryllium has a high boiling point of 2,471 degrees Celsius (4,480 degrees Fahrenheit).
6. Density: Beryllium is one of the least dense metals, with a density of 1.85 grams per cubic centimeter.
7. Specific Gravity: The specific gravity of Beryllium is 1.85, which means it is about 1.85 times heavier than an equal volume of water.
8. Hardness: Beryllium is a hard and brittle metal with a Mohs hardness of 5.5 to 6.
9. Conductivity: Beryllium is an excellent conductor of both electricity and heat.
10. Magnetic Properties: Beryllium is non-magnetic, meaning it does not exhibit magnetic properties.
These physical properties contribute to Beryllium’s usefulness in various applications, particularly in industries where its lightweight, high melting point and excellent conductivity are beneficial.
Physical properties of Lithium
The physical properties of Lithium include:
1. Appearance: Lithium is a soft, silvery-white metal.
2. Color: It has a silver color when freshly cut, but it quickly tarnishes to a grayish color upon exposure to air.
3. Texture: Lithium is a soft and malleable metal that can be easily cut with a knife.
4. Melting Point: The melting point of Lithium is relatively low at 180.5 degrees Celsius (356.9 degrees Fahrenheit).
5. Boiling Point: Lithium has a relatively low boiling point of 1,342 degrees Celsius (2,448 degrees Fahrenheit).
6. Density: Lithium is the least dense solid element, with a density of 0.534 grams per cubic centimeter.
7. Specific Gravity: The specific gravity of Lithium is 0.534, which means it is about 0.534 times heavier than an equal volume of water.
8. Hardness: Lithium could be a moderately delicate metal with a Mohs hardness of 0.6.
9. Conductivity: Lithium is a good conductor of both electricity and heat.
10. Magnetic Properties: Lithium is paramagnetic, meaning it is weakly attracted to magnetic fields but does not retain magnetism when the field is removed.
These physical properties of Lithium contribute to its various applications, particularly in industries such as battery manufacturing, where its low density, high conductivity, and reactivity are advantageous. The softness of Lithium allows for ease of processing and shaping, while its low melting point makes it suitable for certain applications requiring low-temperature operations.
Occurrence and Sources
Occurrence and sources of Beryllium:
1. Natural occurrence: Beryllium may be a moderately uncommon component within the Earth’s outside, happening at a normal concentration of almost 2 to 6 parts per million (ppm). It is not found in its pure form but is typically found in various minerals and ores.
2. Minerals and ores: Beryllium is primarily found in the mineral form of beryl (Be_3Al_2(SiO_3)_6), which is a beryllium aluminum cyclosilicate. Other minerals that contain beryllium include bertrandite (Be_4Si_2O_7(OH)_2) and phenakite (Be_2SiO_4).
3. Sources: The main sources of beryllium are mining operations that extract beryllium-containing minerals. These minerals are ordinarily found in pegmatite veins, granitic rocks, and certain sorts of transformative and aqueous stores. Beryllium can be extracted from the minerals through various refining processes.
Occurrence and sources of Lithium:
1. Natural occurrence: Lithium is the 25th most abundant element in the Earth’s crust, occurring at an average concentration of about 20 ppm. It is present in trace amounts in rocks, soil, and natural brine deposits.
2. Minerals and ores: Lithium is found in various minerals, including spodumene (LiAl(SiO_3)_2), petalite (LiAlSi_4O_10), lepidolite (KLi_2Al(Al, Si)_3O_10(F, OH)_2), and amblygonite (LiAlPO_4(F, OH)).
3. Sources: Lithium is primarily obtained from three main sources:
a. Pegmatite veins and granitic rocks: These rocks contain lithium-bearing minerals like spodumene and petalite. Mining operations extract lithium ore from these sources.
b. Saline brine deposits: Lithium can also be found in certain natural brine deposits, typically associated with lithium-rich salt lakes and salt flats. These brines contain dissolved lithium salts, which can be extracted through evaporation and chemical processes.
c. Lithium-containing clays: Some clays and claystone contain lithium and extraction methods such as acid leaching and solvent extraction are used to recover lithium from these sources.
The production of both beryllium and lithium involves mining and extraction processes to obtain the elements from their respective minerals or ores.
Natural occurrence of Beryllium
Beryllium happens actually within the Earth’s hull but in moderately moo concentrations.
Here are a few keys focus concerning the characteristic event of beryllium:
1. Abundance: Beryllium is considered a moderately uncommon component within the Earth’s hull. It is assessed to have a normal wealth of around 2 to 6 parts per million (ppm) by weight.
2. Minerals and Ores: Beryllium is primarily found in various minerals and ores. The most significant beryllium-containing mineral is beryl (Be₃Al₂SiO₆), which is a cyclosilicate. Other Minerals That Contain Beryllium Incorporate Bertrandite (Be₄Si₂O₇(OH)₂), Phenakite (Be₂SiO₄), and Chrysoberyl (BeAl₂O₄).
3. Geologic Settings: Beryllium-rich minerals are typically found in specific geologic settings. These incorporate pegmatite veins, which are coarse-grained volcanic rocks, as well as certain sorts of granitic rocks. Beryllium deposits can also occur in hydrothermal veins associated with volcanic activity and in some types of metamorphic rocks.
4. Global Distribution: Beryllium deposits are distributed worldwide but are generally more concentrated in specific regions. Major beryllium-producing nations incorporate the Joined together States (essentially in Utah and Colorado), Brazil, China, Mozambique, and Russia. Be that as it may, beryllium isn’t commonly mined as an essential item and is regularly obtained as a byproduct of other mineral mining operations.
5. Extraction: To extract beryllium from its minerals, various extraction processes are employed. These can involve crushing and grinding the ore to liberate beryllium-bearing minerals, followed by physical and chemical methods to separate and refine beryllium compounds.

Whereas beryllium is moderately uncommon within the Earth’s outside, it can be found in certain minerals and metals in particular geologic settings, giving a source for its extraction and consequent utilization in different businesses.
Natural occurrence of Lithium
Lithium occurs naturally in various geological and environmental settings.
Here are some important aspects regarding the natural occurrence of lithium:
1. Abundance: Lithium is a relatively abundant element, but it is dispersed rather than concentrated in high-grade deposits. It has a normal plenitude of around 20 parts per million (ppm) within the Earth’s outside, making it more inexhaustible than components like lead, mercury, and silver.
2. Minerals and Ores: Lithium is primarily found in certain minerals and ores. The Main Lithium-Containing Minerals Include Spodumene (LiAl(SiO₃)₂), Petalite (LiAlSi₄O₁₀), Lepidolite (K(Li,Al)₂-₃(Al,Si)₄O₁₀(F,OH)₂), and Amblygonite (LiAlPO₄(F,OH)). These minerals can contain varying amounts of lithium, with spodumene being the most important commercial source due to its relatively high lithium content.
3. Geologic Settings: Lithium deposits can occur in different geologic settings. They Are Commonly Associated With Pegmatites, Which Are Coarse-Grained Igneous Rocks Formed From Magma. Pegmatites often contain lithium-rich minerals, including spodumene and lepidolite. Lithium Can Also Be Found in Certain Granitic Rocks, as well as in Saline Brine Deposits Associated With Salt Lakes and Salt Flats.
4. Global Distribution: Lithium deposits are distributed worldwide, but some regions have higher concentrations of economically significant deposits. Major lithium-producing countries include Australia, Chile, China, Argentina, and Zimbabwe. These countries possess significant lithium resources and have active mining and production operations.
5. Extraction: The extraction of lithium from minerals and brine deposits involves various techniques. For minerals like spodumene, conventional mining methods such as crushing, grinding, and flotation are used to concentrate the lithium-bearing ores. Brine deposits, on the other hand, involve extracting lithium from underground brine reservoirs through pumping and evaporation processes.
It’s Worth Noting That the Commercial Viability of Lithium Extraction Depends on the Concentration and Purity of Lithium in the Deposits, as well as the Economic and Environmental Considerations Associated With the Extraction Processes.
Health and Safety Considerations
Health and safety considerations for Beryllium:
1. Toxicity: Beryllium and its compounds are highly toxic. Inhalation of Beryllium Dust, Fumes, or Vapors Can Cause a Severe Lung Condition Called Chronic Beryllium Disease (CBD) or Berylliosis. CBD is an incurable and potentially fatal lung disease that can develop even from low levels of beryllium exposure.
2. Skin Contact: Beryllium can also cause some individuals’ skin sensitization and allergic reactions. Direct Skin Contact With Beryllium or Its Compounds Should Be Avoided to Prevent Dermatitis and Other Skin-Related Health Issues.
3. Engineering Controls: Proper engineering controls, such as local exhaust ventilation and enclosed systems, should be in place to minimize airborne beryllium exposure in workplaces where beryllium is handled or processed.
4. Personal Protective Equipment (PPE): Workers handling beryllium or working in areas with potential beryllium exposure should wear appropriate PPE, including respiratory protection (such as respirators with high-efficiency particulate filters), protective clothing, gloves, and safety glasses or goggles.
5. Regulatory Compliance: Workplaces need to comply with local occupational health and safety regulations regarding beryllium handling and exposure limits. These regulations may include permissible exposure limits (PELs), requirements for medical surveillance of workers, and specific control measures.
Health and safety considerations for Lithium:
1. Fire and Explosion Hazard: Lithium metal and certain lithium compounds can pose fire and explosion hazards. Lithium is highly reactive and can react violently with water and air. Proper storage and handling procedures should be followed to prevent fire or explosion incidents.
2. Skin and Eye Contact: Direct contact with metallic lithium can cause skin and eye irritation. Proper personal protective equipment, such as gloves and safety glasses, should be used when handling lithium to prevent skin and eye contact.
3. Electrical Hazards: Lithium batteries, particularly lithium-ion batteries, can store a significant amount of electrical energy. Appropriate safeguards ought to be taken when dealing with or working with lithium batteries to dodge electrical stun or short-circuiting dangers.
4. Thermal Hazards: Lithium batteries can generate heat during the charging and discharging processes. Overheating or thermal runaway of lithium batteries can lead to fire and thermal hazards. Secure charging and capacity hones, as well as warm administration frameworks, ought to be actualized to moderate these dangers.
5. Waste Management: Proper disposal and recycling of lithium batteries are important to prevent environmental contamination. Lithium batteries ought to be reused through suitable reusing programs or arranged in agreement with neighborhood controls to play down natural effects.
It is significant to follow important security rules, directions, and best hones to guarantee the secure dealing with, capacity, and transfer of both beryllium and lithium to secure the well-being and security of laborers and the environment.
Comparison Chart
Sure! Here’s a comparison chart highlighting the key differences between Beryllium and Lithium:
| Property | Beryllium | Lithium |
|---|---|---|
| Chemical Symbol | Be | Li |
| Atomic Number | 4 | 3 |
| Atomic Structure | 2 electrons in the first energy level, 2 electrons in the second energy level | 2 electrons in the first energy level, 1 electron in the second energy level |
| Group | Group 2 (alkaline earth metals) | Group 1 (alkali metals) |
| Period | Period 2 | Period 2 |
| Density | 1.85 g/cm³ | 0.534 g/cm³ |
| Melting Point | 1,287°C (2,349°F) | 180.5°C (356.9°F) |
| Boiling Point | 2,471°C (4,480°F) | 1,342°C (2,448°F) |
| Toxicity | Highly toxic, can cause chronic beryllium disease | Relatively low toxicity, but can cause skin and eye irritation |
| Applications | Aerospace, defense, electronics, nuclear applications | Lithium-ion batteries, pharmaceuticals, glass/ceramic applications |
Please note that this is a simplified comparison, and there may be additional properties or nuances to consider.
Conclusion
Beryllium and lithium possess distinctive properties that differentiate them from each other. While beryllium exhibits higher density, melting point, and rigidity, lithium stands out with its lower density and reactivity. Both elements find valuable applications in various industries, contributing to advancements in technology and energy storage. Understanding the differences between beryllium and lithium is crucial in utilizing their unique properties effectively while considering safety precautions.
