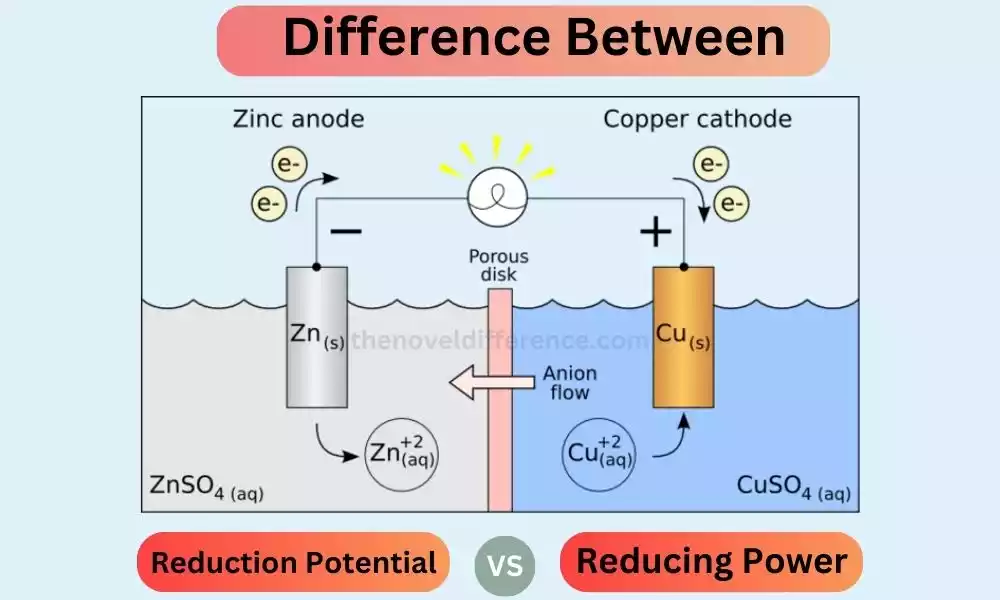
Hey there! Have you ever wondered about the fascinating world of electrochemical reactions? Today, I’m going to shed some light on two important concepts reduction potential and reducing power. They play significant roles in understanding how substances undergo chemical transformations through electron transfer. So, let’s dive in and unravel the mystery!
Why Understanding the Difference Matters
Now, you might be wondering why it’s important to differentiate between reduction potential and reducing power.
Well, let me break it down for you:
Contextual Differences: Reduction potential primarily applies to electrochemical reactions, where the focus is on measuring the potential difference between two electrodes. Reducing power finds its significance in biological systems, where electron transfer is essential for cellular functions. Recognizing the specific contexts in which these terms operate allows us to apply them accurately.
Varied Measurement and Units: Reduction potential is typically measured in volts (V) and is often represented by a standardized potential value. In contrast, reducing power is more challenging to measure directly, as it depends on multiple factors. It can be indirectly assessed by observing the capability of a substance to donate electrons. Being aware of these measurement variations aids in the proper interpretation and utilization of these concepts.
Practical Applications: Understanding the differences between reduction potential and reducing power has practical implications across various fields. The knowledge of reduction potential helps in designing and optimizing electrochemical processes. Understanding reducing power is crucial in biochemical research, where it influences reactions involved in energy production, detoxification, and more.
Definition of Reduction Potential and Reducing Power
Reduction Potential: Reduction potential refers to the tendency of a chemical species to gain electrons and undergo reduction. It represents the ease with which a substance can be reduced in an electrochemical reaction. It’s like a measure of how badly a species wants to gain electrons. The higher the reduction potential, the stronger the desire to be reduced.
To measure reduction potential, scientists use a reference electrode and a working electrode. The difference in electrical potential between these two electrodes determines the reduction potential of the species being studied. The measurement is typically carried out under standard conditions, known as standard reduction potential (E°).
Reducing Power: Now, let’s talk about reducing power. It’s a measure of the ability of a substance to donate electrons and cause the reduction of other species. In other words, reducing power tells us how good a substance is at giving away its electrons to facilitate chemical reactions.
Determining reducing power involves considering factors like the concentration of reducing agents, the availability of electrons, and the ease of electron transfer. Substances with high reducing power are more capable of transferring electrons and causing reductions in other species.
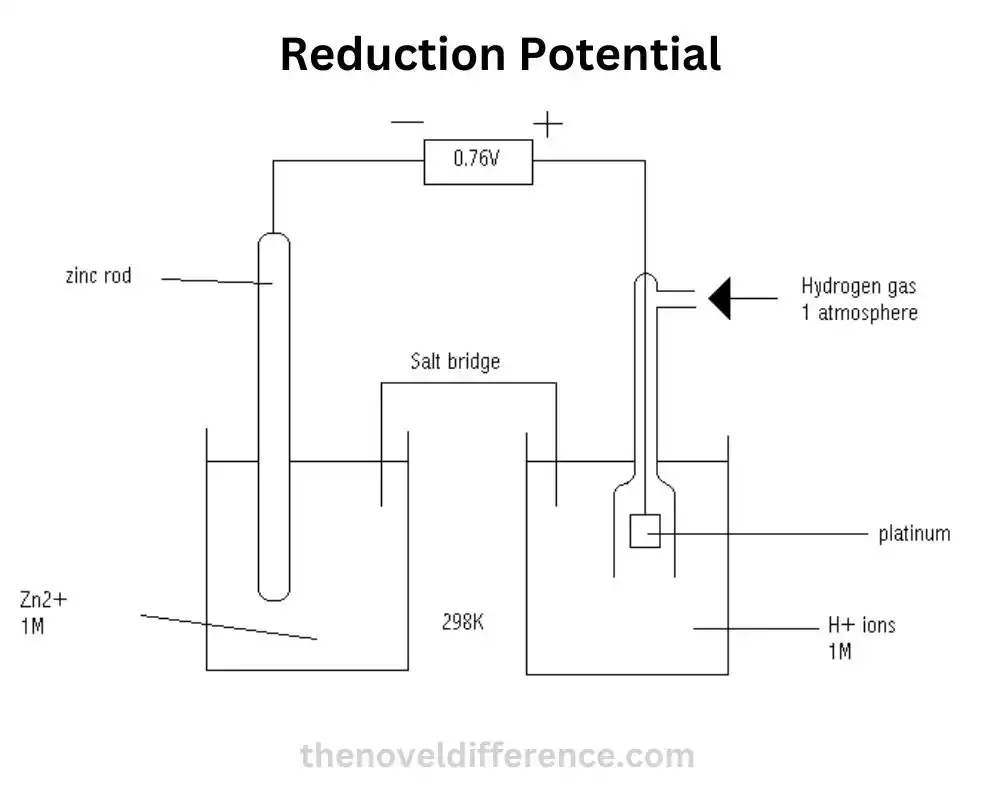
What is Reduction Potential?
Reduction potential, often denoted as E°, is a measure of the tendency of a chemical species to gain electrons and undergo reduction. It is measured in volts (V) and represents the electric potential difference between the species being reduced and the standard hydrogen electrode (SHE). The standard hydrogen electrode is considered to have a reduction potential of 0 volts by convention.
Step-by-Step Explanation:
1. Redox Reactions: Redox reactions involve the transfer of electrons between chemical species. The species that loses electrons is oxidized, while the species that gains electrons is reduced. Reduction potential helps us determine which species has a greater tendency to gain electrons and undergo reduction.
2. Comparison with the Standard Hydrogen Electrode: The standard hydrogen electrode (SHE) is often used as a reference point in determining reduction potential. It is an electrode immersed in a solution of 1M hydrogen ions with hydrogen gas at a pressure of 1 atm. By convention, the reduction potential of the SHE is considered 0 volts. Reduction potentials are measured relative to this standard.
3. Positive and Negative Reduction Potentials: Reduction potentials can be positive or negative. A positive reduction potential indicates that a species has a greater tendency to gain electrons and be reduced compared to the SHE. Conversely, a negative reduction potential indicates a lower tendency to gain electrons. The greater the positive reduction potential, the stronger the oxidizing agent. The more negative the reduction potential, the stronger the reducing agent.
4. Relationship with Equilibrium: Reduction potential is closely related to the equilibrium constant (K) of a redox reaction. A positive reduction potential corresponds to a forward reaction that favors reduction, while a negative reduction potential favors oxidation. The relationship is given by the Nernst equation, which allows us to calculate the reduction potential under non-standard conditions.
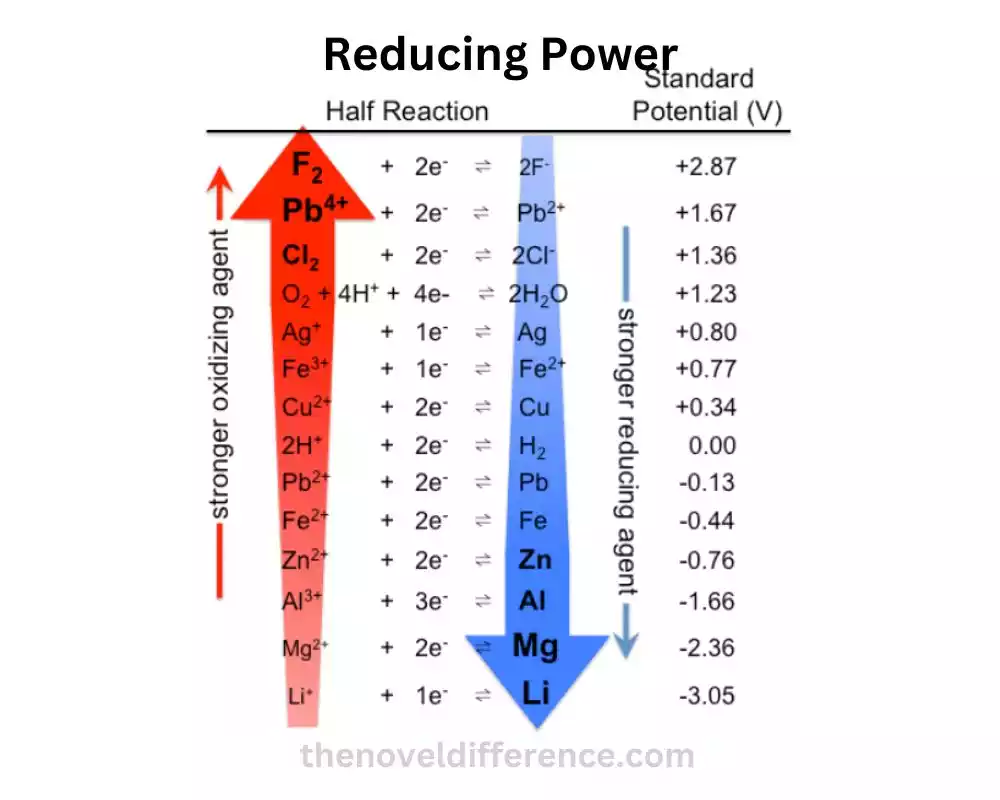
What is Reducing Power?
Reducing power is the measure of a substance’s ability to donate electrons and undergo oxidation in a chemical reaction. It signifies the strength of a reducing agent, which is a substance that readily gives up electrons. Think of reducing power as the generosity of a compound to share its electrons with other species.
Step-by-Step Explanation:
1. Redox Reactions: Redox reactions involve the transfer of electrons between chemical species. The substance that donates electrons is oxidized, while the substance that accepts electrons is reduced. Reducing power allows us to identify which substances have a greater tendency to donate electrons and act as reducing agents.
2. Electron Donors: Substances with high reducing power act as efficient electron donors. They readily release electrons, making them suitable for driving various chemical reactions. Examples of common electron donors include reducing sugars, such as glucose, and certain metals like sodium or magnesium.
3. Oxidation States: Understanding oxidation states is crucial in grasping reducing power. The higher the oxidation state of a substance, the lower its reducing power. Conversely, substances with lower oxidation states tend to possess stronger reducing power. This is because higher oxidation states indicate a higher number of electron deficiencies, making the substance more eager to donate electrons.
4. Presence of Reducing Agents: Reducing agents are compounds that are known for their high reducing power. They can reduce other substances by donating electrons. Common examples of reducing agents include sodium borohydride (NaBH4) and ascorbic acid (vitamin C). These substances are widely used in various chemical and biological processes.
Difference between Reduction Potential and Reducing Power
The difference between reduction potential and reducing power can be summarized as follows:
1. Definition:
• Reduction Potential: Reduction potential is a quantitative measure of the tendency of a chemical species to gain electrons and undergo a reduction in a redox reaction. It quantifies the thermodynamic driving force for reduction compared to a reference electrode.
• Reducing Power: Reducing power is a qualitative measure of the ability of a substance or system to donate electrons and act as a reducing agent in a redox reaction. It indicates the strength and capacity of a substance to transfer electrons.
2. Nature of Measurement:
• Reduction Potential: Reduction potential is measured in volts (V) and represents the potential energy difference between the species being reduced and the reference electrode. It is a quantitative measurement of the thermodynamic tendency for reduction.
• Reducing Power: Reducing power is a qualitative assessment and does not have a specific unit of measurement. It is evaluated based on factors such as the concentration of reducing agents, the stability of the oxidized form, and the availability of electrons for transfer.
3. Focus:
• Reduction Potential: Reduction potential is primarily used in electrochemical and physical chemistry contexts. It helps predict the direction, spontaneity, and feasibility of redox reactions. It focuses on the thermodynamic aspect of electron transfer.
• Reducing Power: Reducing power is often discussed in biochemical and biological systems. It is relevant to the reactivity and electron-donating capability of substances. It focuses on the qualitative aspect of electron transfer and the ability to act as a reducing agent.
4. Application:
• Reduction Potential: Reduction potential is applied in electrochemical cells, corrosion prevention, metal extraction, and other industrial processes. It is also relevant in predicting chemical reactivity and understanding redox reactions in various fields.
• Reducing Power: Reducing power is particularly important in biological systems, such as cellular metabolism, photosynthesis, and enzymatic reactions. It helps evaluate the electron transfer processes and the electron-donating capability of substances in biochemical reactions.
Reduction potential provides a quantitative measure of the thermodynamic tendency for reduction, primarily used in electrochemical and physical chemistry contexts. Reducing power is a qualitative measure of the ability to donate electrons, focusing on the reactivity and electron-donating capability of substances, especially in biological systems.
Practical Applications
Reduction potential and reducing power have numerous practical applications across various fields. The reduction potential is utilized in electroplating, corrosion prevention, and battery technology. Understanding the reduction potential of different materials helps engineers design effective electrochemical systems.
Reducing power is crucial for energy production, metabolism, and various cellular processes. The transfer of electrons mediated by reducing agents is vital for the synthesis of ATP (adenosine triphosphate), the energy currency of cells. Understanding reducing power aids in studying diseases, drug development, and bioengineering applications.
Conclusion
Understanding the difference between reduction potential and reducing power is vital in comprehending the intricate world of electrochemical reactions. Reduction potential measures the likelihood of a substance accepting electrons while reducing power measures its ability to donate or transfer electrons.
By grasping these concepts, we gain insights into the spontaneity of reactions, electron transfer processes, and the behavior of substances in various applications. From energy storage to chemical synthesis, reduction potential and reducing power have significant implications across a range of fields.
So, the next time you encounter these terms, remember their distinct focuses. Reduction potential reveals the inclination to accept electrons while reducing power highlights the ability to donate electrons. Armed with this knowledge, you’re one step closer to unraveling the captivating mysteries of electrochemistry!