Definition of Addition and Substitution Reaction
addition reaction:
An addition reaction in chemistry refers to any chemical process where two or more reactants combine into a single product. This reaction involves the addition of atoms, groups, or molecules to a reactant or a substrate, forming a new compound.
Addition reactions typically occur between unsaturated compounds, such as alkenes or alkynes, and an electrophile or a nucleophile. In an addition reaction, the pi bond in the unsaturated compound is broken, allowing the addition of atoms or groups to the carbon atoms involved in the double or triple bond.
Addition reactions are commonly encountered in organic chemistry and play a crucial role in various synthetic and industrial processes.
substitution reaction:
Substitution reaction refers to any chemical process wherein one element or functional group in a molecule is replaced with another atom, group, or ion. In a substitution reaction, the substituent takes the place of an existing atom or group, forming a new compound. Substitution reactions can occur in various types of compounds, including organic and inorganic substances.
Substitution reactions fall into two primary categories: nucleophilic substitution (SN) and electrophilic substitution.
- Nucleophilic substitution (SN): In nucleophilic substitution reactions, a nucleophile, which is an electron-rich species, replaces a leaving group in a molecule. The nucleophile donates a pair of electrons to the atom it is attacking, forming a new bond. Nucleophilic substitution reactions are commonly observed in organic chemistry, particularly in reactions involving alkyl halides.
- Electrophilic substitution: In electrophilic substitution reactions, an electrophile, which is an electron-deficient species, replaces an atom or group in a molecule. The electrophile seeks electrons to complete its octet or stabilize its positive charge, and it attacks a region of high electron density in the molecule. Electrophilic substitution reactions often occur in aromatic compounds, such as benzene, where the electrophile substitutes a hydrogen atom in the aromatic ring.
Substitution reactions have various applications in organic synthesis, pharmaceutical development, and industrial processes. Understanding the mechanisms and factors influencing substitution reactions is essential for designing efficient chemical reactions and predicting the behavior of different compounds.
Importance of understanding the differences between addition and substitution reactions
Understanding the differences between addition and substitution reactions is of significant importance for several reasons:
- Reaction Prediction: Differentiating between addition and substitution reactions allows chemists to predict the products and mechanisms of chemical reactions accurately. By recognizing the specific characteristics and requirements of each reaction type, chemists can anticipate the outcome of a given reaction and design appropriate reaction conditions.
- Reaction Optimization: Understanding the disparities between addition and substitution reactions helps in optimizing reaction conditions for desired outcomes. Factors such as temperature, solvent choice, and catalyst selection can be tailored based on the reaction type to enhance reaction efficiency and selectivity.
- Synthetic Strategies: The knowledge of addition and substitution reactions aids in designing effective synthetic strategies. Chemists can choose the appropriate reaction type to introduce specific functional groups or modify existing ones, facilitating the synthesis of complex molecules and the development of new materials.
- Mechanistic Understanding: Differentiating between addition and substitution reactions provides insights into the underlying mechanisms involved. It helps in comprehending the step-by-step processes, bond-breaking and bond-forming events, and electron flow during reactions. This knowledge enables chemists to propose and validate reaction mechanisms accurately.
- Industrial Applications: Addition and substitution reactions have widespread industrial applications.Understanding the differences between addition reactions such as hydrogenation or polymerization and substitution reactions such as nucleophilic substitution or aromatic substitution is vital to designing efficient processes in pharmaceutical, petrochemical and polymer industries.
- Biological Relevance: Understanding the distinctions between addition and substitution reactions is crucial for studying and developing drugs, understanding enzymatic reactions, and analyzing biochemical pathways. Differentiating between these reaction types helps in understanding how various functional groups are modified or exchanged within biological systems.
- Safety and Hazards: Recognizing the differences between addition and substitution reactions is crucial for safety considerations in handling and working with reactive chemicals. Different reaction types may require different precautions, as they can exhibit distinct reactivity, byproduct formation, or potential hazards.
A comprehensive understanding of the differences between addition and substitution reactions enables chemists to make informed decisions, design efficient syntheses, and apply these concepts to various scientific and industrial fields.
Addition Reactions
Addition reactions are chemical processes where two or more reactants combine into one product. In an addition reaction, atoms, groups, or molecules are added to a reactant or substrate, forming a new compound. Addition reactions typically occur between unsaturated compounds, such as alkenes or alkynes, and an electrophile or a nucleophile.
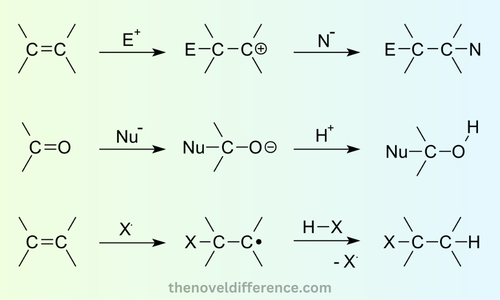
Here are some key aspects of addition reactions:
- Unsaturated Substrates: Addition reactions commonly involve unsaturated compounds that possess double or triple bonds. These unsaturated substrates are more reactive due to the presence of π (pi) bonds, which can be broken during the addition process.
- Electrophiles and Nucleophiles: Addition reactions involve the interaction of an electrophile and a nucleophile. An electrophile is an electron-deficient species that seeks electrons, while a nucleophile is an electron-rich species that donates a pair of electrons. The electrophile attacks the π bond of the unsaturated substrate, leading to the addition of atoms or groups.
- Reaction Mechanism: The mechanism of addition reactions can vary depending on the specific reaction and the reactants involved. Two common mechanisms are stepwise (also known as a carbocation mechanism) and concerted (also known as a pericyclic or concerted mechanism).
- Catalysts: Some addition reactions require the presence of catalysts to facilitate the reaction. Catalysts can enhance the rate of the reaction or provide an alternative reaction pathway, leading to more efficient and selective product formation.
Examples of Addition Reactions:
a. Hydrogenation: Addition of hydrogen to an unsaturated hydrocarbon, typically an alkene or alkyne, leading to its conversion into an alkane.
b. Halogenation: Addition of chlorine or bromine as an unsaturated compound to create a halogenated product.
c. Hydration: Addition of water to an unsaturated compound, leading to the formation of alcohol.
d. Polymerization: Repeated addition of monomer units to form a polymer chain.
Understanding addition reactions is crucial for synthetic chemists as they provide a wide range of possibilities for the construction of new compounds, the modification of functional groups, and the development of industrially relevant processes.
Definition and characteristics of addition reactions
Definition:
An addition reaction is defined as any chemical process by which two or more reactants combine into one product. It involves the addition of atoms, groups, or molecules to a reactant or substrate, forming a new compound.
Characteristics of Addition Reactions:
- Reactants: Addition reactions typically involve unsaturated compounds as reactants. Unsaturated compounds contain double or triple bonds, such as alkenes or alkynes, which provide the necessary pi (π) electrons for the addition process. These reactive bonds act as sites for the addition of atoms or groups.
- Bond Breaking and Formation: Addition reactions involve the breaking of existing bonds and the formation of new bonds. The π bond in the unsaturated compound is typically broken, allowing atoms or groups to add to the carbon atoms involved in the double or triple bond. The addition can occur at one or both ends of the multiple bonds.
- Electrophiles and Nucleophiles: Addition reactions involve the interaction of electrophiles and nucleophiles. An electrophile is an electron-deficient species that is attracted to electron-rich regions and seeks electrons. A nucleophile is an electron-rich species that donates a pair of electrons to form a new bond. Electrophiles and nucleophiles play crucial roles in the addition process by facilitating the transfer of electrons.
- Reaction Mechanisms: The mechanism of addition reactions can vary depending on the specific reaction and the reactants involved. Two common mechanisms are the stepwise (or carbocation) mechanism and the concerted (or pericyclic) mechanism. The stepwise mechanism involves the formation of an intermediate carbocation, while the concerted mechanism involves simultaneous bond-breaking and bond-forming events.
- Catalysts: Addition reactions can be influenced by the presence of catalysts. Catalysts are substances that increase the rate of a reaction without being consumed in the process. They can enhance the reactivity of the reactants or provide an alternative reaction pathway, leading to more efficient and selective product formation.
- Product Formation: Addition reactions result in the formation of a single product. Product structure is determined by both the nature of reactants used as well as regioselectivity (preferring particular locations of addition) and stereoselectivity of reactions, both of which influence which sites or spatial arrangements they prefer for reactions. The addition of atoms or groups can occur in a syn or anti-orientation, depending on the reaction conditions and the stereochemistry of the reactants.
Addition reactions are important in organic chemistry and have wide-ranging applications in synthesis, pharmaceuticals, materials science, and other fields. Understanding their characteristics helps in predicting reaction outcomes, designing synthetic routes, and developing new compounds.
Substitution Reactions
Substitution reactions are chemical processes in which an atom or functional group in a molecule is changed into another element such as an atom, group, ion etc. In a substitution reaction, the substituent takes the place of an existing atom or group, forming a new compound.
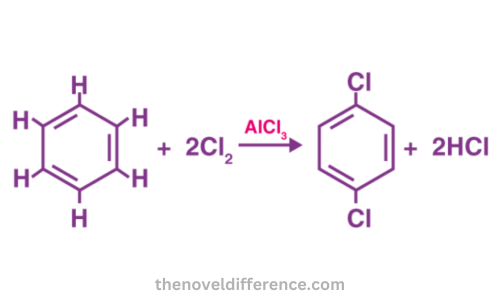
Here are some key aspects of substitution reactions:
- Reactants: Substitution reactions often involve saturated compounds, such as alkanes or alkyl halides, as reactants. These compounds are relatively stable and do not possess double or triple bonds.
- Nucleophiles and Electrophiles: Substitution reactions can be categorized into nucleophilic substitution (SN) and electrophilic substitution reactions based on the nature of the attacking species. In nucleophilic substitution, a nucleophile, an electron-rich species, replace a leaving group in the molecule. In electrophilic substitution, an electrophile, an electron-deficient species, replaces an atom or group.
- Reaction Mechanisms: The mechanism of substitution reactions depends on the specific reaction and the type of substitution occurring. Nucleophilic substitution techniques typically employed include the following two approaches for nucleophilic replacements: (SN1) unimolecular nucleophilic substitution and (2) bimolecular nucleophilic substitution for replacements whereas electrophilic aromatic substitution is utilized when making electrophilic replacements.
- Leaving Group: In substitution reactions, the leaving group is the atom or group that is displaced from the molecule. It can be an atom or a functional group that has a weak bond to the rest of the molecule and is more electronegative than the attacking species. The leaving group departs with its pair of electrons during the substitution process.
- Rate-Determining Step: In most substitution reactions, the rate-determining step can often determine the rate of reaction. In nucleophilic substitutions, the rate-determining step can vary depending on whether it is an SN1 or SN2 reaction. In electrophilic aromatic substitutions, the rate-determining step involves the attack of the electrophile on the aromatic ring.
- Factors Influencing Reaction Outcome: Various factors, such as the nature of the nucleophile or electrophile, the leaving group, the solvent, and the reaction conditions, can influence the rate and outcome of substitution reactions. Steric hindrance, electronic effects, and solvent polarity are particularly important in determining the feasibility and selectivity of the substitution process.
Substitution reactions play a crucial role in organic chemistry, pharmaceutical development, and industrial processes. Understanding their mechanisms and factors influencing their outcomes is essential for designing efficient chemical reactions, predicting the behavior of different compounds, and synthesizing new compounds with desired properties.
Definition and characteristics of substitution reactions
Definition:
Substitution reactions refer to chemical processes in which an atom or functional group in a molecule is replaced with another atom, group, or ion. In a substitution reaction, the substituent takes the place of an existing atom or group, forming a new compound.
Characteristics of Substitution Reactions:
- Reactants: Substitution reactions often involve saturated compounds, such as alkanes or alkyl halides, as reactants. These compounds typically do not possess double or triple bonds and have relatively stable chemical structures.
- Nucleophiles and Electrophiles: Substitution reactions can be classified into nucleophilic substitution (SN) and electrophilic substitution reactions based on the nature of the attacking species. In nucleophilic substitution, a nucleophile, an electron-rich species, replaces a leaving group in the molecule. In electrophilic substitution, an electrophile, an electron-deficient species, replaces an atom or group in the molecule.
- Reaction Mechanisms: The mechanism of substitution reactions can vary depending on the specific reaction and the type of substitution occurring. Some common mechanisms include SN1 (unimolecular nucleophilic substitution), SN2 (bimolecular nucleophilic substitution), and electrophilic aromatic substitution.
- Leaving Group: In substitution reactions, a leaving group is the atom or group that is displaced from the molecule during the reaction. The leaving group departs from the molecule along with its pair of electrons. It is typically more electronegative than the attacking species and has a weak bond to the rest of the molecule.
- Rate-Determining Step: In substitution reactions, the slowest step often serves as the rate-determining step and governs its rate of progress. In nucleophilic substitutions, the rate-determining step can vary depending on whether it is an SN1 or SN2 reaction. In electrophilic aromatic substitutions, the rate-determining step involves the attack of the electrophile on the aromatic ring.
- Factors Influencing Reaction Outcome: Several factors can influence the outcome of substitution reactions, including the nature of the nucleophile or electrophile, the leaving group, the solvent, temperature, and steric effects. These factors can affect the reaction rate, selectivity, and regiochemistry, leading to different substitution products.
Substitution reactions have significant applications in organic synthesis, pharmaceutical development, and industrial processes. Understanding their characteristics, mechanisms, and factors that influence their outcomes is essential for designing efficient chemical reactions, predicting product formation, and controlling reaction selectivity.
Differences between Addition and Substitution Reactions
Addition Reactions:
- Definition: Addition reactions involve the combination of two or more reactants to form a single product, where atoms, groups, or molecules are added to a reactant or substrate.
- Reactants: Addition reactions typically involve unsaturated compounds, such as alkenes or alkynes, as reactants. These compounds contain double or triple bonds that provide sites for addition.
- Bond Breaking and Formation: Addition reactions involve the breaking of π (pi) bonds in unsaturated compounds and the formation of new σ (sigma) bonds with added atoms or groups.
- Mechanism: Addition reactions can proceed through stepwise (carbocation) or concerted (pericyclic) mechanisms, depending on the specific reaction and reactants involved.
- Electrophiles and Nucleophiles: Addition reactions usually involve interactions between electrophilic (electron-deficient species) and nucleophilic species, both having electron-rich environments for reactions. Electrophiles seek electrons and attack the π bond, while nucleophiles donate a pair of electrons for the formation of new bonds.
- Catalysts: Some addition reactions require the presence of catalysts to facilitate the reaction by enhancing the rate or providing an alternative reaction pathway.
Substitution Reactions:
Substitution reactions involve changing one or more atoms or functional groups within molecules to generate new compounds, in turn leading to substitution reactions and thus compound formation.
- Reactants: Substitution reactions typically involve saturated compounds, such as alkanes or alkyl halides, as reactants. These compounds lack double or triple bonds.
- Breaking and Forming Bonds: Substitution reactions involve breaking bonds between the substituent and host molecule and creating new ones with a newly introduced substitute, followed by the formation of the newly introduced substitution agent.
- Mechanism: Substitution reactions can occur through various mechanisms, such as nucleophilic substitution (SN1 or SN2) or electrophilic aromatic substitution, depending on the specific reaction and reactants involved.
- Nucleophiles and Electrophiles: Substitution reactions involve interactions between electron-rich nucleophiles (such as uniforms) and electrophiles (electron-deficient species). Nucleophiles replace a leaving group, while electrophiles replace an atom or group.
- Leaving Group: Substitution reactions require the presence of a leaving group, which is displaced from the molecule. The leaving group is typically more electronegative than the attacking species and departs along with its pair of electrons.
Key differences between addition and substitution reactions lie in their reactants, the type of bond changes occurring, their mechanisms and any role played by nucleophiles/electrophiles.
Addition reactions involve the addition of atoms/groups to unsaturated compounds, while substitution reactions involve the replacement of atoms/groups in saturated compounds.
Definition and concept
The concept of addition and substitution reactions refers to two fundamental types of chemical reactions that occur between molecules or compounds.
Addition Reactions:
Addition reactions involve the combination of multiple reactants into one single product. In an addition reaction, atoms, groups, or molecules are added to a reactant or substrate, forming a new compound. Typically, addition reactions occur with unsaturated compounds that possess double or triple bonds, such as alkenes or alkynes.
The general form of an addition reaction can be represented as follows:
A + B → AB
Example of Addition Reactions: Adding hydrogen (H2) to an alkene to form an alkane is a classic example of an addition reaction.
Substitution Reactions:
Substitution reactions involve the replacement of an atom or functional group in a molecule by another atom, group, or ion. In a substitution reaction, the substituent takes the place of an existing atom or group, forming a new compound. Substitution reactions often occur with saturated compounds, such as alkanes or alkyl halides.
The general form of a substitution reaction can be represented as follows:
A + B-C → A-C + B
Example: The substitution of a halogen (X) in an alkyl halide by a nucleophile (Nu) is a common example of a substitution reaction.
Both addition and substitution reactions involve reactants undergoing chemical transformations that lead to new chemical bonds forming between their constituent molecules, creating new compounds.
The specific reaction conditions, mechanisms, and factors influencing the reaction outcome can vary depending on the reactants involved and the type of reaction (addition or substitution).
Organic chemists understand that understanding addition and substitution reactions is central to organic chemistry as these processes play a vital role in various chemical processes including synthesis, drug development, materials science and many more fields of study.
Addition vs Substitution Reaction in Tabular Form
Here is a comparison between addition and substitution reactions in tabular form:
| Aspect | Addition Reactions | Substitution Reactions |
|---|---|---|
| Definition | Two or more reactants combine to form a single product by adding atoms, groups, or molecules to a reactant or substrate. | An atom or functional group within a molecule can be replaced by another atom, group, or ion to produce an entirely new compound. |
| Reactants | Unsaturated compounds, such as alkenes or alkynes, which contain double or triple bonds. | Saturated compounds, such as alkanes or alkyl halides, which lack double or triple bonds. |
| Bond Changes | Breaking of π (pi) bonds in unsaturated compounds and formation of new σ (sigma) bonds with added atoms or groups. | The breakage of bonds between the substituent and other parts of the molecule followed by the formation of new ones with its arrival is known as substitution. |
| Mechanism | Stepwise (carbocation) or concerted (pericyclic) mechanisms. | Various mechanisms like nucleophilic substitution (SN1 or SN2) or electrophilic aromatic substitution. |
| Attack Species | Electrophiles or nucleophiles. | Electrophiles or nucleophiles for electrophilic substitutions; for nucleophilic substitutions respectively. |
| Leaving Group | No leaving group involved. | Presence of a leaving group, which is displaced from the molecule during the reaction. |
| Example | Addition of hydrogen (H2) to an alkene to form an alkane. | Substitution of a halogen (X) in an alkyl halide by a nucleophile (Nu). |
| Importance | Addition reactions are important in synthetic chemistry, allowing the construction of new compounds and the modification of functional groups. | Substitution reactions are crucial for organic synthesis, pharmaceutical development, and industrial processes, providing a means to replace specific atoms or groups in molecules. |
Understanding the differences between addition and substitution reactions helps chemists predict reaction outcomes, design synthetic routes, and develop new compounds for various applications.
Applications and Importance
Applications and Importance of Addition and Substitution Reactions:
Addition Reactions:
- Synthesis of Organic Compounds: Addition reactions are at the forefront of developing various organic compounds. They are used to introduce new functional groups, modify existing functional groups, and build complex molecular structures.
- Polymerization: Addition reactions are central to polymerization processes, where monomers undergo repeated addition reactions to form polymers. Plastic, fibers, coatings, and adhesives are just a few uses of polymeric materials produced from this technology.
- Pharmaceutical Development: Addition reactions are utilized in pharmaceutical synthesis to introduce specific functional groups and create pharmacologically active compounds. They are essential in the development of drugs and medicinal compounds.
- Material Science: Addition reactions are employed in the synthesis of materials with specific properties, such as coatings, resins, and specialty chemicals. Engineered materials play an essential part in the design and production of materials used across numerous industries.
Substitution Reactions:
- Drug Discovery and Development: Substitution reactions play a critical role in medicinal chemistry, enabling the introduction of desired functional groups or replacing existing groups in drug molecules. These reactions help optimize drug properties, enhance efficacy, and reduce unwanted side effects.
- Environmental Remediation: Substitution reactions are frequently employed during environmental remediation to replace hazardous or toxic compounds with less hazardous, less toxic alternatives. This is important in cleaning up contaminated soil, water, and air.
- Organic Synthesis: Substitution reactions are fundamental in organic synthesis, allowing chemists to selectively replace specific atoms or functional groups in molecules. This allows the development of novel compounds, modifications of existing molecules and novel synthetic routes.
- Industrial Processes: Substitution reactions find applications in various industrial processes, including the production of fine chemicals, dyes, fragrances, and agrochemicals. They are also employed in the synthesis of polymers and specialty materials used in industrial sectors.
Understanding the mechanisms and applications of addition and substitution reactions is vital for designing efficient chemical processes, developing new materials and drugs, and advancing various areas of scientific research.
These reactions provide chemists with versatile tools to manipulate molecular structures and create compounds with desired properties and functions.
Conclusion
Addition and substitution reactions are two fundamental types of chemical reactions that occur between molecules or compounds. Addition reactions involve the combination of reactants to form a single product by adding atoms, groups, or molecules to a substrate. Substitution reactions, on the other hand, involve the replacement of an atom or functional group in a molecule by another atom, group, or ion.
Understanding the differences between addition and substitution reactions is crucial in various fields, including organic synthesis, pharmaceutical development, material science, and industrial processes.
Addition reactions are important for constructing new compounds, modifying functional groups, and polymerization processes. Substitution reactions play a significant role in replacing specific atoms or groups in molecules, drug discovery and development, environmental remediation, and organic synthesis.
By comprehending the mechanisms, factors influencing outcomes, and applications of addition and substitution reactions, chemists can design efficient chemical processes, develop new materials and drugs, and advance scientific research in diverse areas.
