Explore the key dissimilarities between dry ashing and wet digestion methods in analytical chemistry. Understand their applications, advantages, and limitations. Discover which technique suits your specific research needs.
Introduction
Sample preparation plays a vital role in extracting valuable information from diverse substances. Among the numerous techniques employed, dry ashing and wet digestion have emerged as common and effective methods for preparing samples for analysis. These processes enable scientists to break down complex matrices, facilitate the extraction of analytes, and enhance the accuracy and reliability of subsequent measurements.
We will delve into the intricacies of dry ashing and wet digestion, exploring their principles, advantages, limitations, and applications. By understanding the nuances of these techniques, scientists can make informed decisions when selecting the most suitable sample preparation approach for their analytical endeavors. So, let us embark on this enlightening journey to unravel the difference between dry ashing and wet digestion and gain insights into their respective roles in the fascinating world of analytical chemistry.
What is Dry Ashing?
Dry ashing, also known as dry combustion or dry oxidation, is a sample preparation technique employed in analytical chemistry to facilitate the decomposition of organic matter and the subsequent determination of inorganic components. This method involves subjecting a sample to high temperatures in a controlled environment to promote the combustion and oxidation of organic materials, leaving behind an ash residue containing the inorganic constituents of interest.
The process of dry ashing typically begins with the selection of an appropriate sample, which can vary widely depending on the analytical objectives. The sample is carefully weighed and placed into a crucible or a combustion boat, which is then introduced into a high-temperature furnace or muffle furnace. The temperature is gradually increased to initiate the combustion process, usually in the range of 400-800 degrees Celsius, and maintained for a specified period to ensure complete oxidation.
During the heating and combustion process, organic compounds present in the sample undergo thermal decomposition, vaporization, and combustion, leaving behind a residue primarily composed of inorganic salts, metals, minerals, or other non-volatile components. This residue, often referred to as the “ash,” represents the inorganic fraction of the original sample and serves as the basis for subsequent analytical measurements.
Dry ashing offers several advantages in sample preparation. Firstly, it helps preserve the integrity of the sample by avoiding chemical interactions or losses during the decomposition process. Additionally, the method minimizes the risk of contamination since no chemical reagents are added to the sample. Furthermore, dry ashing applies to a wide range of sample types, including solid materials, plant tissues, biological samples, and environmental samples.
Dry ashing does have some limitations. Certain elements, particularly volatile or easily reducible species, may be lost during the combustion process, leading to incomplete removal or inaccurate quantification. The technique can also be challenging when dealing with complex matrices containing high organic content, as the complete oxidation of organic materials may require higher temperatures or longer heating times. Careful method development and optimization are often necessary to overcome these challenges and ensure accurate results.
Dry ashing finds applications in various fields of analytical chemistry, including environmental analysis, geological and mineral analysis, food and agricultural analysis, and pharmaceutical and biomedical analysis. By enabling the extraction and quantification of inorganic components, dry ashing plays a crucial role in understanding the composition, quality, and potential environmental impact of diverse samples.

We will explore wet digestion, another widely employed sample preparation technique, and delve into its principles, advantages, and applications, further expanding our knowledge of analytical chemistry’s sample preparation toolbox.
Principles and steps involved in dry ashing
Dry ashing is a sample preparation technique that relies on the principles of combustion and oxidation to decompose organic matter and obtain inorganic components. The process involves several key steps to ensure the effective and complete conversion of the sample into ash residue.
Let’s explore the principles and steps involved in dry ashing:
1. Selection of an Appropriate Sample: Before embarking on dry ashing, it is essential to choose a representative sample that aligns with the analytical objectives. The sample may include solid materials, plant tissues, biological samples, or environmental samples.
2. Weighing and Preparing the Sample: The selected sample is accurately weighed to determine the initial mass, which is crucial for subsequent calculations and analysis. It is essential to handle the sample with care to avoid contamination and ensure accurate results.
3. Placement in a Crucible or Combustion Boat: The weighed sample is transferred into a suitable container, such as a crucible or a combustion boat. These vessels are typically made of materials that can withstand high temperatures without interacting with the sample or contributing to the ash residue.
4. Introduction to a High-Temperature Furnace: The crucible or combustion boat containing the sample is carefully placed into a high-temperature furnace or muffle furnace. These furnaces provide a controlled environment with elevated temperatures necessary for the combustion process.
5. Gradual Heating: The temperature within the furnace is gradually increased to initiate the combustion process. This gradual heating helps prevent sudden reactions, ensures uniform heating throughout the sample, and minimizes the risk of sample loss or spattering.
6. Complete Oxidation: Once the desired temperature is reached, the sample is subjected to prolonged heating at the specified temperature. The objective is to achieve complete oxidation of the organic matter present in the sample, converting it into gaseous compounds and leaving behind the inorganic components as ash.
7. Cooling and Handling: After the heating period, the furnace is gradually cooled down before removing the crucible or combustion boat containing the ash residue. It is crucial to handle the container and ash residue with care to avoid contamination and loss of the ash during transfer.
8. Determination of Ash Residue: The ash residue obtained from dry ashing is typically a mixture of inorganic compounds, minerals, metals, or salts. Depending on the analytical objectives, the ash residue may be further processed or directly subjected to subsequent analytical techniques to determine the content and composition of the inorganic components.
Throughout the dry ashing process, it is essential to consider factors such as temperature, heating time, and the nature of the sample to achieve optimal results. Method development and optimization may be necessary to address challenges specific to the sample matrix or target analytes.
Dry ashing offers a reliable means of decomposing organic matter and extracting inorganic components from diverse sample types. It is important to acknowledge its limitations, such as the potential loss of volatile species and challenges with complex matrices. Understanding these principles and steps involved in dry ashing allows scientists to harness the technique effectively and make informed decisions in analytical chemistry research and analysis.
Advantages of dry ashing
Dry ashing, as a sample preparation technique in analytical chemistry, offers several advantages that make it a valuable tool in various research and analysis applications.
Let’s explore some of the key advantages of dry ashing:
1. Preservation of Sample Integrity: Dry ashing preserves the integrity of the sample by avoiding chemical interactions or losses during the decomposition process. Since no chemical reagents are added, there is minimal risk of introducing contamination or altering the sample composition. This advantage is particularly crucial when analyzing samples with sensitive or reactive components.
2. Minimal Contamination Risks: Dry ashing involves subjecting the sample to high temperatures, which can effectively eliminate or reduce contamination risks associated with reagents or vessels. The absence of chemical digestion agents minimizes the potential introduction of impurities or unwanted compounds during sample preparation.
3. Applicability to Various Sample Types: Dry ashing is widely applicable to different sample types, including solid materials, plant tissues, biological samples, and environmental samples. It offers versatility and can be adapted to suit diverse analytical objectives, making it a valuable technique across various fields of research.
4. Preservation of Inorganic Components: By effectively decomposing organic matter, dry ashing selectively preserves the inorganic components present in the sample. This facilitates subsequent analysis and quantification of the target elements or compounds of interest. The resulting ash residue serves as a concentrated and purified form of the inorganic fraction.
5. Compatibility with Analytical Techniques: The ash residue obtained from dry ashing is typically amenable to a wide range of analytical techniques for further quantification and characterization. It can be readily subjected to techniques such as atomic absorption spectroscopy (AAS), inductively coupled plasma optical emission spectroscopy (ICP-OES), or X-ray fluorescence (XRF) analysis, among others.
6. Wide Range of Applications: Dry ashing finds applications in various fields of analytical chemistry. It is commonly utilized in environmental analysis to determine the presence of heavy metals, minerals, or pollutants in soils, sediments, and other environmental samples. Additionally, it is employed in geological and mineral analysis, food and agricultural analysis, pharmaceutical and biomedical analysis, and more.
While dry ashing offers numerous advantages, it is important to consider the specific characteristics of the sample and the analytical objectives. The technique may have limitations, such as the potential loss of volatile compounds or challenges with complex matrices. Method development and optimization are often necessary to ensure accurate and reliable results.
Dry ashing provides researchers with a reliable and versatile method for decomposing organic matter and extracting inorganic components from a wide range of samples. Its preservation of sample integrity, minimal contamination risks, and compatibility with various analytical techniques make it a valuable tool in analytical chemistry research and analysis.
What is Wet Digestion?
Wet digestion is a sample preparation technique widely employed in analytical chemistry to break down complex matrices and facilitate the extraction of analytes for subsequent analysis. This method involves the use of chemical reagents to dissolve or digest the sample, resulting in a solution or suspension containing the target analytes of interest.
The principles of wet digestion revolve around the chemical reactions that occur between the sample and the digestion reagents. These reactions can involve processes such as acid dissolution, oxidation, reduction, or complexation, depending on the nature of the sample and the analytes being targeted.The steps involved in wet digestion can vary depending on the specific method and the analytical objectives.
A typical wet digestion process includes the following key steps:
1. Selection of Digestion Reagents: Based on the nature of the sample and the analytes of interest, appropriate digestion reagents are chosen. These reagents can include acids (such as nitric acid, hydrochloric acid, or sulfuric acid), oxidizing agents, reducing agents, or complexing agents. The selection of reagents is crucial to ensure efficient digestion and solubilization of the analytes.
2. Sample Preparation: The sample is carefully weighed or measured to determine the initial mass or volume. Depending on the nature of the sample, it may undergo pre-treatment steps such as grinding, homogenization, or filtration to enhance its digestibility and remove interfering substances.
3. Addition of Digestion Reagents: The selected digestion reagents are added to the sample in appropriate quantities. The mixture is typically heated, stirred, or subjected to other conditions to facilitate the digestion process. The specific digestion parameters (such as temperature, time, and agitation) are optimized to ensure complete and efficient digestion.
4. Chemical Reactions and Digestion: The digestion reagents interact with the sample, leading to chemical reactions that dissolve, oxidize, reduce, or complex the analytes. These reactions break down the complex matrix, solubilizing the target analytes and transforming them into a solution or suspension suitable for subsequent analysis.
5. Filtration or Separation: After the digestion process is complete, the digested mixture may undergo filtration or other separation techniques to remove insoluble residues or particulate matter. This step ensures that the resulting solution or suspension is free from unwanted solid materials.
6. Analyte Extraction and Analysis: The digested solution or suspension containing the target analytes is further processed, as required, to extract and concentrate the analytes of interest. Various analytical techniques, such as spectroscopy, chromatography, or elemental analysis methods, can be applied to quantify and characterize the extracted analytes.
Wet digestion offers several advantages in sample preparation. It allows for the efficient decomposition of complex samples, enhancing the recovery of target analytes and facilitating their subsequent analysis. The technique is compatible with various analytical methods, making it versatile for different research areas, including environmental analysis, food and agricultural analysis, pharmaceutical and biomedical analysis, and more.
Wet digestion also has limitations to consider. The use of digestion reagents introduces the possibility of contamination or chemical interferences, and there is a risk of analyte loss or degradation during the digestion process. Careful method development, optimization, and quality control measures are necessary to mitigate these limitations and ensure accurate and reliable results.
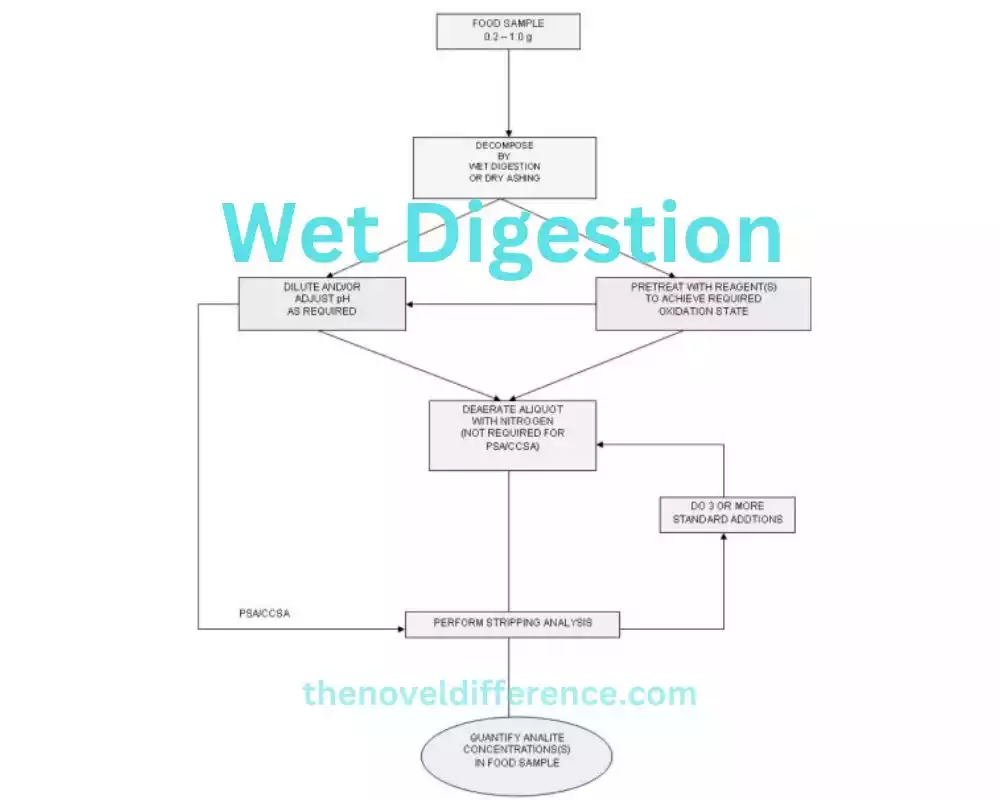
We will further explore the advantages, limitations, and applications of wet digestion, providing a comprehensive understanding of its role in analytical chemistry.
Principles and steps involved in wet digestion
Wet digestion is a sample preparation technique that involves the use of chemical reagents to dissolve or digest a sample, allowing for the extraction and subsequent analysis of analytes of interest. The principles and steps involved in wet digestion can vary depending on the specific method and analytical objectives.
The following are general principles and steps commonly associated with wet digestion:
1. Selection of Digestion Reagents: The choice of digestion reagents depends on the nature of the sample matrix and the analytes to be extracted. Commonly used reagents include acids (such as nitric acid, hydrochloric acid, or sulfuric acid), oxidizing agents, reducing agents, or complexing agents. The selection of appropriate reagents is crucial for efficient digestion and analyte solubilization.
2. Sample Preparation: The sample is carefully prepared by weighing or measuring the desired amount. Depending on the sample type, it may undergo pre-treatment steps such as grinding, homogenization, or filtration to enhance digestion efficiency and remove interfering substances.
3. Addition of Digestion Reagents: The digestion reagents are added to the sample in the appropriate quantities. The mixture is typically heated, stirred, or subjected to other conditions to promote the digestion process. Parameters such as temperature, time, and agitation are optimized to ensure complete digestion.
4. Chemical Reactions and Digestion: The digestion reagents react with the sample matrix, leading to chemical transformations that dissolve, oxidize, reduce, or complex analytes of interest. These reactions break down the complex matrix, solubilizing the analytes and converting them into a solution or suspension suitable for further analysis.
5. Filtration or Separation: Once digestion is complete, the digested mixture may undergo filtration or other separation techniques to remove insoluble residues or particulate matter. This step ensures that the resulting solution or suspension is free from unwanted solid materials.
6. Analyte Extraction and Analysis: The digested solution or suspension containing the target analytes is further processed to extract and concentrate the analytes of interest. This can involve techniques such as liquid-liquid extraction, solid-phase extraction, or other isolation methods depending on the nature of the analytes and the desired analytical method. The extracted analytes are then subjected to appropriate analytical techniques, such as spectroscopy, chromatography, or elemental analysis, for quantification and characterization.
It is important to note that the specific digestion conditions and methods may vary depending on the sample matrix, the analytes being targeted, and the desired analytical outcome. Method optimization and validation are essential to ensure accurate and reliable results.
Wet digestion offers several advantages in sample preparation, allowing for efficient decomposition of complex samples and enhanced extraction of analytes. It is widely applied in various fields, including environmental analysis, food and agricultural analysis, pharmaceutical and biomedical analysis, and more.
Wet digestion also has limitations. The use of digestion reagents introduces the possibility of contamination or interference, and there is a risk of analyte loss or degradation during the digestion process. Careful selection of reagents, method optimization, and quality control measures are necessary to mitigate these limitations and ensure accurate and reliable results.
By understanding the principles and steps involved in wet digestion, scientists can make informed decisions in selecting and implementing this technique for their specific analytical needs.
Advantages of wet digestion
Wet digestion, as a sample preparation technique in analytical chemistry, offers several advantages that make it a valuable tool for the extraction and analysis of analytes.
Let’s explore some of the key advantages of wet digestion:
1. Enhanced Analyte Extraction: Wet digestion facilitates the efficient extraction of analytes from complex sample matrices. The digestion reagents employed in the process break down the sample matrix, dissolving and solubilizing the analytes of interest. This enhances the recovery and availability of the analytes for subsequent analysis, leading to improved sensitivity and accuracy.
2. Versatility in Analyte Types: Wet digestion can be applied to a wide range of analyte types, including metals, minerals, organic compounds, and biological substances. The technique is adaptable to different sample matrices and can handle diverse analytes, making it suitable for various fields of analysis.
3. Wide Applicability: Wet digestion finds applications in various research areas, including environmental analysis, food and agricultural analysis, pharmaceutical and biomedical analysis, and more. It allows for the extraction and quantification of analytes in complex sample matrices, providing valuable information for quality control, environmental monitoring, and scientific research.
4. Control of Digestion Parameters: Wet digestion allows for precise control of digestion parameters such as temperature, time, and reagent concentrations. This enables optimization of the digestion process to ensure complete digestion, minimize analyte losses, and mitigate interferences. The ability to fine-tune the digestion conditions enhances the reliability and accuracy of the analytical results.
5. Simultaneous Decomposition of Complex Matrices: Wet digestion is particularly advantageous when dealing with complex sample matrices that contain various components or interferences. The digestion reagents effectively break down the matrix, dissolving unwanted components and allowing for the selective extraction of the analytes of interest. This simplifies the subsequent analysis by reducing interferences and improving analyte specificity.
6. Compatibility with Various Analytical Techniques: The digested solution or suspension obtained from wet digestion is compatible with a wide range of analytical techniques. Depending on the nature of the analytes and the desired analytical outcome, the extracted analytes can be quantified and characterized using techniques such as spectroscopy, chromatography, or elemental analysis methods.
7. Detection of Low Concentration Analytes: Wet digestion can be particularly useful for the analysis of analytes present at low concentrations in a sample. By enhancing the extraction efficiency and concentration of the analytes, wet digestion improves the detectability of low-level analytes, enabling their accurate quantification and characterization.
8. Quality Control and Standardization: Wet digestion allows for standardized sample preparation procedures, enabling better quality control in analytical laboratories. By following established wet digestion protocols, laboratories can ensure consistent and reliable results, facilitating comparisons between different samples and studies.
It is important to note that wet digestion also has limitations and considerations, such as the potential for interferences, contamination risks, and the need for careful selection and optimization of digestion reagents. With proper method development, optimization, and quality control measures, these limitations can be minimized, and the advantages of wet digestion can be fully realized.
Wet digestion offers enhanced analyte extraction, versatility in analyte types, wide applicability, and control of digestion parameters. Its compatibility with various analytical techniques and ability to handle complex sample matrices make it a valuable technique in analytical chemistry for extracting and analyzing analytes of interest.
Difference between Dry Ashing and Wet Digestion
Dry ashing and wet digestion are two common sample preparation techniques used in analytical chemistry, particularly for the extraction and analysis of inorganic components from complex sample matrices. While both methods aim to break down the sample matrix and facilitate analyte extraction.
There are fundamental differences between dry ashing and wet digestion. Let’s explore these differences:
1. Principle:
• Dry Ashing: Dry ashing involves subjecting the sample to high temperatures in a controlled environment, typically in a muffle furnace. The high temperatures cause the organic components of the sample to combust, leaving behind an inorganic residue or ash. The ash residue is then further processed for analysis.
• Wet Digestion: Wet digestion, on the other hand, relies on chemical reactions between the sample and digestion reagents to dissolve or digest the matrix. The digestion reagents, which can include acids or other chemicals, react with the sample, breaking down the matrix and facilitating the extraction of analytes into a solution or suspension.
2. Sample Matrix:
• Dry Ashing: Dry ashing is primarily suitable for solid or semi-solid samples. It is commonly used for organic-rich samples, such as plant tissues, biological samples, or environmental samples containing organic matter.
• Wet Digestion: Wet digestion can be applied to a wide range of sample matrices, including solids, liquids, and even complex matrices. It is particularly useful for samples that require solubilization or digestion of the matrix to extract the analytes of interest.
3. Reagents:
• Dry Ashing: Dry ashing does not involve the addition of digestion reagents. It relies solely on high temperatures for organic combustion, leaving behind an ash residue.
• Wet Digestion: Wet digestion requires the addition of specific digestion reagents, such as acids, oxidizing agents, reducing agents, or complexing agents. These reagents react with the sample matrix to dissolve or digest it, aiding in analyte extraction.
4. Preservation of Sample Integrity:
• Dry Ashing: Dry ashing preserves the inorganic components of the sample while removing organic matter through combustion. It selectively concentrates the inorganic fraction, making it suitable for subsequent analysis of inorganic analytes.
• Wet Digestion: Wet digestion involves chemical reactions that break down the sample matrix and dissolve or digest organic and inorganic components. It allows for the extraction of both organic and inorganic analytes, but the preservation of sample integrity can vary depending on the digestion reagents used.
5. Contamination Risks:
• Dry Ashing: Dry ashing minimizes contamination risks since it does not involve the addition of digestion reagents. There is a potential for contamination from external sources during the ashing process.
• Wet Digestion: Wet digestion carries a higher risk of contamination due to the addition of digestion reagents. Careful selection of reagents and stringent quality control measures are necessary to minimize contamination risks.
6. Analytical Techniques:
• Dry Ashing: The ash residue obtained from dry ashing is typically compatible with techniques such as atomic absorption spectroscopy (AAS), inductively coupled plasma optical emission spectroscopy (ICP-OES), or X-ray fluorescence (XRF) analysis, which can be used to quantify and characterize the inorganic analytes.
• Wet Digestion: The digested solution or suspension obtained from wet digestion can be subjected to a wide range of analytical techniques, including spectroscopy, chromatography, or elemental analysis, depending on the nature of the analytes and the desired analytical outcome.
It’s important to note that the selection between dry ashing and wet digestion depends on various factors, including the sample type, the analytes of interest, and the specific analytical objectives. Each method has its advantages and limitations, and the choice should
Applications of Dry Ashing and Wet Digestion
Dry ashing and wet digestion are sample preparation techniques commonly employed in analytical chemistry. They find applications in various fields and are particularly useful for the extraction and analysis of inorganic components from complex sample matrices.
Let’s explore some of the key applications of dry ashing and wet digestion:
Applications of Dry Ashing:
1. Environmental Analysis: Dry ashing is widely used in environmental analysis to determine the levels of inorganic contaminants in soil, sediments, air particulates, and other environmental samples. It allows for the quantification of elements such as heavy metals, trace elements, and minerals, providing valuable information for environmental monitoring and assessment.
2. Geology and Mining: Dry ashing is employed in geological studies and mining operations to analyze rock samples and ore concentrates. It aids in the identification and quantification of valuable minerals, as well as the determination of impurities and trace elements present in geological materials.
3. Archaeology and Anthropology: Dry ashing is utilized in the analysis of archaeological and anthropological samples, such as bone, teeth, and pottery fragments. It helps in determining the elemental composition and mineral content of these materials, providing insights into past human activities, diets, and cultural practices.
4. Pharmaceutical Analysis: Dry ashing is applied in the analysis of pharmaceutical formulations to determine the levels of inorganic impurities, such as heavy metals. It ensures compliance with regulatory standards and helps assess the safety and quality of pharmaceutical products.
Applications of Wet Digestion:
1. Environmental Analysis: Wet digestion is extensively used in environmental analysis to extract and analyze inorganic components from various sample matrices. It is employed for the determination of metals, metalloids, nutrients, and other inorganic contaminants in water, soil, sediment, and biological samples.
2. Food and Agricultural Analysis: Wet digestion plays a crucial role in the analysis of food and agricultural samples. It aids in the extraction and quantification of essential nutrients, toxic elements, and trace elements in food products, animal feeds, crops, and agricultural soils.
3. Pharmaceutical and Biomedical Analysis: Wet digestion is utilized in pharmaceutical and biomedical analysis to extract and quantify inorganic components from drug formulations, biological tissues, and fluids. It helps assess the elemental composition, purity, and potential toxicities of pharmaceutical products or investigate the distribution of elements in biological samples.
4. Material Science and Quality Control: Wet digestion is employed in material science and quality control processes to analyze the composition and purity of materials such as metals, alloys, ceramics, and polymers. It assists in assessing the presence of impurities, determining elemental compositions, and verifying product quality.
5. Forensic Analysis: Wet digestion finds application in forensic analysis for the extraction and analysis of inorganic components from various forensic samples. It aids in the detection and quantification of elements and compounds relevant to forensic investigations, such as gunshot residue analysis or toxicology studies.
These are just a few examples of the wide-ranging applications of dry ashing and wet digestion in analytical chemistry. The techniques play a crucial role in extracting and analyzing inorganic components, providing valuable information for research, quality control, environmental monitoring, and various scientific disciplines.
Conclusion
The difference between dry ashing and wet digestion lies in their fundamental principles, sample states, processes, and applications. Dry ashing is commonly employed for solid samples and utilizes high temperatures for combustion, while wet digestion can handle solid, liquid, and gaseous samples by utilizing strong acids for dissolution.
Each technique has its advantages and limitations, making them suitable for specific analytical objectives. Dry ashing is often preferred for its simplicity and applicability to a wide range of sample matrices. Wet digestion, on the other hand, provides better analyte recovery and is effective for samples with complex matrices.
Understanding the dissimilarities between dry ashing and wet digestion allows analytical chemists to select the most appropriate method for their research or analysis requirements. By making an informed choice, researchers can ensure accurate and reliable results in their analytical investigations.