Introduction
When it comes to understanding and balancing redox reactions, two commonly used methods are the Ion Electron and Oxidation Number Methods. These techniques provide a systematic approach to determining the oxidation states and balance equations in chemical reactions involving electron transfer. We’ll dive into the subtleties of each strategy, highlight their contrasts, and investigate the variables to consider when selecting the fitting approach for understanding redox responses.
Definition of Ion Electron Method and Oxidation Number Method
Ion Electron Method: The Particle Electron Strategy, too known as the Half-Reaction Strategy or the Half-Reaction Ion-Electron Adjusting Strategy, could be a procedure utilized to adjust redox (reduction-oxidation) responses. This method involves breaking down the overall redox equation into two separate half-reactions: one representing the reduction half and the other representing the oxidation half. By balancing these half-reactions individually, the overall equation can be balanced.
In the Ion Electron Method, the key steps include:
1. Identifying the species undergoing reduction and oxidation.
2. Assign oxidation numbers to each element in the reaction.
3. Write separate half-reactions, one for reduction and one for oxidation, by showing the species gaining or losing electrons.
4. Balancing the atoms other than oxygen and hydrogen in each half-reaction.
5. Balancing the charges in each half-reaction by adding electrons to the appropriate side.
6. Ensuring that the number of electrons transferred in the oxidation half-reaction matches the number of electrons gained in the reduction half-reaction.
7. Combining the balanced half-reactions to obtain the overall balanced redox equation.
The Ion Electron Method is particularly useful for complex redox reactions that cannot be easily balanced using other methods. It provides a systematic approach to balancing equations by focusing on the transfer of electrons between species involved in the reaction.
Oxidation Number Method:
The Oxidation Number Strategy, moreover known as the Oxidation State Strategy or the Alter in Oxidation Number Strategy, is another method utilized to adjust redox responses. This method relies on the concept of oxidation numbers, which are assigned to atoms within compounds to indicate their electron distribution and the degree of oxidation or reduction.
The steps involved in the Oxidation Number Method are as follows:
1. Assign oxidation numbers to each element in the reaction, following specific rules and guidelines.
2. Identifying the species undergoing oxidation and reduction by observing the change in oxidation numbers.
3. Calculate the change in oxidation number for each element in the reaction.
4. Balancing the changes in oxidation numbers by adding coefficients to the appropriate compounds or species.
5. Balancing the atoms other than oxygen and hydrogen in the equation.
6. Ensuring that the overall charge is balanced by adding electrons as necessary.
7. Verifying that the number of electrons lost in the oxidation process equals the number of electrons gained in the reduction process.
8. Confirm the overall balanced equation.
The Oxidation Number Method provides a simpler approach to balancing redox equations, particularly when dealing with straightforward reactions. It focuses on analyzing the changes in the oxidation states of elements to achieve balance in the overall equation.
What is Ion Electron Method?
The Particle Electron Strategy, moreover known as the Half-Reaction Strategy or the Half-Reaction Ion-Electron Adjusting Strategy, could be a strategy utilized to adjust redox (reduction-oxidation) responses. Redox responses include the transfer of electrons between species, coming about within the oxidation of one species and the decrease of another.
The Ion Electron Method involves breaking down the overall redox equation into two separate half-reactions: one representing the reduction half and the other representing the oxidation half. By balancing these half-reactions individually, the overall equation can be balanced.
The key steps involved in the Ion Electron Method are as follows:
1. Identification of the species undergoing reduction and oxidation: Determine which species is being reduced (gaining electrons) and which is being oxidized (losing electrons) in the reaction.
2. Assignment of oxidation numbers: Assign oxidation numbers to each element in the reaction based on specific rules. Oxidation numbers indicate the charge an atom would have if electrons were assigned completely to the more electronegative element in a compound.
3. Writing separate half-reactions: Construct separate half-reactions for the reduction and oxidation processes. In the reduction half-reaction, the species gaining electrons is written, while in the oxidation half-reaction, the species losing electrons is written.
4. Balancing atoms: Balance the number of atoms other than oxygen and hydrogen in each half-reaction by adding coefficients as necessary.
5. Balancing charges: Balance the charges in each half-reaction by adding electrons to the appropriate side. Electrons are added to the side of the half-reaction where reduction is occurring and subtracted from the side where oxidation is occurring.
6. Equalizing electrons: Ensure that the number of electrons transferred in the oxidation half-reaction matches the number of electrons gained in the reduction half-reaction. This step involves multiplying the half-reactions by appropriate coefficients to achieve electron equality.
7. Combining the half-reactions: Add the balanced half-reactions together, canceling out common terms, to obtain the overall balanced redox equation.
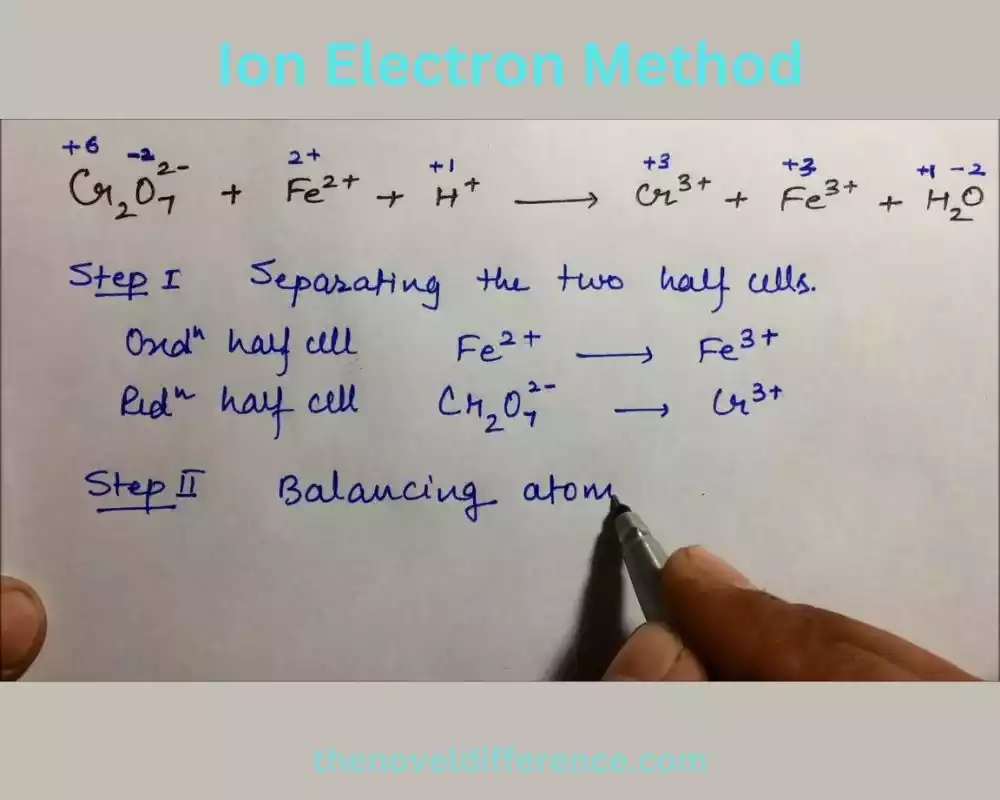
The Ion Electron Method provides a systematic approach to balancing redox equations, particularly for complex reactions that cannot be easily balanced using other methods. By focusing on the transfer of electrons and breaking down the reaction into separate reduction and oxidation processes, the Ion Electron Method allows for precise balancing of redox equations.
Steps Involved in the Ion Electron Method
The Particle Electron Strategy, moreover known as the Half-Reaction Strategy or the Half-Reaction Ion-Electron Adjusting Strategy, could be a strategy utilized to adjust redox (reduction-oxidation) responses. It involves breaking down the overall redox equation into separate reduction and oxidation half-reactions and balancing them individually.
Here are the steps involved in the Ion Electron Method:
1. Identify the species undergoing reduction and oxidation: Determine which species is being reduced (gaining electrons) and which is being oxidized (losing electrons) in the reaction. This step is crucial in determining the appropriate half-reactions.
2. Assign oxidation numbers: Assign oxidation numbers to each element in the reaction. Oxidation numbers are assigned based on specific rules and guidelines that depend on the electronegativity and bonding characteristics of the elements involved. The oxidation numbers indicate the charge an atom would have if electrons were assigned completely to the more electronegative element in a compound.
3. Write separate half-reactions: Construct separate half-reactions for the reduction and oxidation processes. In the reduction half-reaction, write the species gaining electrons. In the oxidation half-reaction, write the species losing electrons. Make sure to include the correct stoichiometric coefficients for each species based on the changes in their oxidation states.
4. Balance atoms other than oxygen and hydrogen: Balance the number of atoms other than oxygen and hydrogen in each half-reaction by adding coefficients as necessary. This step ensures that the number of atoms on both sides of the half-reaction equation is equal.
5. Balance charges: Balance the charges in each half-reaction by adding electrons to the appropriate side. Electrons are added to the side of the half-reaction where reduction is occurring and subtracted from the side where oxidation is occurring. The goal is to ensure that the total charge is balanced on both sides of the half-reaction.
6. Equalize electrons: Adjust the coefficients of the half-reactions to equalize the number of electrons transferred. Duplicate the half-reactions by fitting coefficients so that the number of electrons picked up within the lessening half-reaction matches the number of electrons misplaced within the oxidation half-reaction. This step ensures that the overall charge is conserved.
7. Combine the half-reactions: Add the balanced half-reactions together, canceling out common terms, to obtain the overall balanced redox equation. Ensure that all atoms, charges, and electrons are properly balanced on both sides of the equation.
By following these steps, the Ion Electron Method allows for the systematic balancing of redox equations, particularly for complex reactions where other methods may be less effective. It provides a clear approach to balancing equations by focusing on the transfer of electrons in separate reduction and oxidation half-reactions.
Advantages and limitations
Advantages of the Ion Electron Method:
1. Systematic approach: The Ion Electron Method provides a systematic and step-by-step approach to balancing redox equations. It breaks down the overall reaction into separate reduction and oxidation half-reactions, making the balancing process more organized and manageable.
2. Flexibility in handling complex reactions: The Ion Electron Method is particularly useful for balancing complex redox reactions that involve multiple species and intermediate steps. It allows for the individual balancing of each half-reaction, which simplifies the overall process for intricate reactions.
3. Conserves the Law of Conservation of Charge and Mass: The Ion Electron Method ensures that the overall redox equation satisfies the Law of Conservation of Charge and the Law of Conservation of Mass. By balancing both charge and mass in each half-reaction, the method helps maintain the fundamental principles of chemistry.
Limitations of the Ion Electron Method:
1. Challenges in determining oxidation states accurately: Assigning oxidation states to elements in some compounds can be challenging, especially for complex molecules or when multiple oxidation states are possible. Determining the correct oxidation states is crucial for writing the correct half-reactions and balancing the equation accurately.
2. Limited applicability for certain reactions: While the Ion Electron Method is suitable for many redox reactions, there may be cases where other methods, such as the Oxidation Number Method, are more appropriate or efficient. Some reactions may require alternative approaches depending on their characteristics.
3. Time-consuming for large-scale reactions: Balancing large-scale redox reactions using the Ion Electron Method can be time-consuming and require careful calculations. The method involves multiple steps, including assigning oxidation numbers, balancing atoms, and equalizing charges and electrons. This complexity can make the process more time-intensive, especially for extensive or complex reactions.
It’s worth noting that the advantages and limitations of the Ion Electron Method should be considered in the context of the specific redox reaction being balanced. Different methods may be more suitable depending on the complexity, scale, and specific requirements of the reaction at hand.
What is the Oxidation Number Method?
The Oxidation Number Strategy, moreover known as the Oxidation State Strategy or the Alter in Oxidation Number Strategy, could be a method utilized to adjust redox (reduction-oxidation) responses. It involves assigning oxidation numbers to elements in compounds and using changes in oxidation numbers to balance the overall equation. The method focuses on analyzing the changes in the oxidation states of elements during the reaction.
Here’s an overview of the Oxidation Number Method:
1. Assign oxidation numbers: Begin by assigning oxidation numbers to each element in the reaction. Oxidation numbers are assigned based on specific rules and guidelines. These rules take into account the electronegativity and bonding characteristics of the elements involved. Oxidation numbers indicate the charge an atom would have if electrons were assigned completely to the more electronegative element in a compound.
2. Identify the species undergoing oxidation and reduction: Determine which species is being oxidized (losing electrons) and which is being reduced (gaining electrons) in the reaction. This identification is crucial in the subsequent steps of the Oxidation Number Method.
3. Calculate changes in oxidation numbers: Determine the change in oxidation number for each element in the reaction. Compare the initial oxidation numbers of the elements in the reactants with their final oxidation numbers in the products. The changes in oxidation numbers reflect the transfer of electrons during the redox reaction.
4. Balance changes in oxidation numbers: Balance the changes in oxidation numbers by adding coefficients to the appropriate compounds or species. These coefficients represent the stoichiometric ratios needed to equalize the changes in oxidation numbers.
5. Balance atoms other than oxygen and hydrogen: Balance the number of atoms (excluding oxygen and hydrogen) on both sides of the equation by adjusting the coefficients of the compounds or species involved. This step guarantees that the number of particles is the same on both sides of the condition.
6. Balance charges: Adjust the coefficients of species or add electrons to balance the charges on both sides of the equation. This step ensures that the overall charge is conserved during the redox reaction.
7. Verify the balance: Check that the number of atoms, charges, and electrons is balanced on both sides of the equation. Ensure that the sum of the oxidation changes in the reactants equals the sum in the products.

By following these steps, the Oxidation Number Method allows for the systematic balancing of redox equations by considering the changes in the oxidation numbers of the elements. It provides a straightforward approach to balancing equations, particularly for simpler redox reactions where the changes in oxidation states are more apparent.
Sequential Steps of the Oxidation Number Method
The Oxidation Number Method, also known as the Oxidation State Method or the Change in Oxidation Number Method, is a systematic approach used to balance redox (reduction-oxidation) equations. The method involves several sequential steps to balance the equation based on changes in oxidation numbers.
Here are the step-by-step procedures of the Oxidation Number Method:
1. Assign oxidation numbers: Start by assigning oxidation numbers to each element in the reactants and products of the redox equation. Oxidation numbers are assigned based on specific rules that consider the electronegativity and bonding characteristics of the elements. This step establishes the initial oxidation states of the elements involved.
2. Identify the oxidized and reduced species: Determine which species is undergoing oxidation (increase in oxidation number) and which is undergoing reduction (decrease in oxidation number). This identification helps in constructing the appropriate half-reactions.
3. Calculate the change in oxidation number: Determine the change in oxidation number for each element in the reaction. Compare the initial oxidation number of each element in the reactants with its final oxidation number in the products. The difference in oxidation numbers represents the change due to electron transfer during the redox reaction.
4. Balance changes in oxidation numbers: Balance the changes in oxidation numbers by adding coefficients to the appropriate compounds or species in the equation. The coefficients represent the stoichiometric ratios required to equalize the changes in oxidation numbers.
5. Balance atoms other than oxygen and hydrogen: Adjust the coefficients of compounds or species other than oxygen and hydrogen to balance the number of atoms on both sides of the equation. This step ensures that the equation satisfies the law of conservation of mass.
6. Balance oxygen atoms: Balance the number of oxygen atoms by adding water (H2O) molecules to the side of the equation requiring additional oxygen atoms. The coefficient of water is determined by the number of oxygen atoms needed.
7. Balance hydrogen atoms: Balance the number of hydrogen atoms by adding hydrogen ions (H+) or hydronium ions (H3O+) to the side of the equation requiring additional hydrogen atoms. The coefficient of hydrogen ions or hydronium ions is determined by the number of hydrogen atoms needed.
8. Balance charges: Adjust the coefficients of species or add electrons (e-) to balance the charges on both sides of the equation. Electrons are included to the side with the next positive charge and subtracted from the side with the next negative charge.
9. Verify and simplify the equation: Check that the number of atoms, charges, and electrons is balanced on both sides of the equation. Make any necessary adjustments to ensure the equation is properly balanced. Finally, simplify the equation by canceling out any common terms.
By following these sequential steps, the Oxidation Number Method enables the systematic and accurate balancing of redox equations based on the changes in oxidation numbers of the elements involved in the reaction.
Benefits and drawbacks
Benefits of the Oxidation Number Method:
1. Systematic approach: The Oxidation Number Method provides a systematic step-by-step approach to balancing redox equations. It follows a logical sequence of assigning oxidation numbers, identifying oxidized and reduced species, and balancing changes in oxidation numbers. This systematic approach makes it easier to balance equations, especially for complex redox reactions.
2. Clear consideration of electron transfer: The Oxidation Number Method explicitly considers the transfer of electrons during a redox reaction. By focusing on changes in oxidation numbers, it highlights the electron transfer between species involved in the reaction. This helps in understanding the underlying concept of oxidation and reduction.
3. Applicable to various types of redox reactions: The Oxidation Number Method can be applied to a wide range of redox reactions, including reactions involving elements, ions, and compounds. It is particularly useful for reactions where changes in oxidation states are more apparent, making it suitable for many common redox scenarios.
Drawbacks of the Oxidation Number Method:
1. Challenging for complex compounds: Balancing redox equations using the Oxidation Number Method can be challenging for complex compounds or reactions involving multiple oxidation states of elements. Determining the correct oxidation numbers for elements in such compounds requires a good understanding of oxidation number rules and the structure of the molecule. In some cases, assigning oxidation numbers accurately can be difficult.
2. Limited applicability for certain reactions: While the Oxidation Number Method is effective for many redox reactions, there may be cases where other methods, such as the Ion Electron Method, are more appropriate or efficient. Some reactions may require alternative approaches, such as the use of oxidation-reduction half-reactions or the use of redox table values.
3. Lack of consideration for reaction conditions: The Oxidation Number Method focuses solely on balancing the changes in oxidation numbers and does not account for reaction conditions or factors such as pH, temperature, and pressure. In certain cases, these factors may have an impact on the redox reaction and its balancing requirements.
4. Complex equations can be time-consuming: Balancing complex redox equations using the Oxidation Number Method can be time-consuming, especially when multiple steps and adjustments are necessary. Large-scale reactions or reactions involving numerous species may require extensive calculations and trial-and-error to achieve a balanced equation.
It’s important to note that the advantages and drawbacks of the Oxidation Number Method should be considered in the context of the specific redox reaction being balanced. Different methods may be more suitable depending on the complexity, scale, and specific requirements of the reaction.
Difference between the Ion Electron and Oxidation Number Methods
The Ion Electron and Oxidation Number Methods are two different approaches used to balance redox (reduction-oxidation) equations. While both methods aim to achieve a balanced equation, they differ in their approach and focus.
Here are the key differences between the Ion Electron and Oxidation Number Methods:
1. Approach:
• Ion Electron Method: This method focuses on breaking down the overall redox equation into separate reduction and oxidation half-reactions. It involves balancing the electron transfer in each half-reaction and then combining them to obtain the balanced equation. The method emphasizes the transfer of electrons and the conservation of charge.
• Oxidation Number Method: This method centers around analyzing changes in the oxidation states (oxidation numbers) of elements in the reactants and products. It involves assigning oxidation numbers, calculating the changes in oxidation numbers, and balancing the equation based on these changes. The method highlights the changes in oxidation states and the conservation of atoms.
2. Balancing Approach:
• Ion Electron Method: The equation is balanced by adjusting stoichiometric coefficients and adding electrons to ensure the conservation of charge and mass. The focus is on balancing both the number of atoms and the charge on each side of the equation.
• Oxidation Number Method: The Oxidation Number Method balances the equation by adjusting stoichiometric coefficients and adding water (H2O) molecules or hydrogen ions (H+) to balance oxygen and hydrogen atoms, respectively. The emphasis is on balancing the number of atoms and the overall charge on each side of the equation.
3. Consideration of Half-Reactions:
• Ion Electron Method: The Ion Electron Method explicitly considers the reduction and oxidation half-reactions as separate entities. It balances each half-reaction individually, accounting for the transfer of electrons in the redox process. The half-reactions are then combined to obtain the overall balanced equation.
• Oxidation Number Method: The Oxidation Number Method does not explicitly separate the equation into reduction and oxidation half-reactions. It focuses on analyzing the changes in oxidation numbers of elements throughout the reaction and balancing the equation based on these changes.
4. Applicability:
• Ion Electron Method: The Ion Electron Method is particularly useful for balancing complex redox reactions involving multiple species, intermediate steps, or reactions occurring in acidic or basic conditions. It provides a systematic approach to balancing equations, especially in more intricate scenarios.
• Oxidation Number Method: The Oxidation Number Method applies to a wide range of redox reactions, including simpler reactions and those involving changes in oxidation states that are more apparent. It provides a straightforward approach to balancing equations based on changes in oxidation numbers.
It’s critical to note that both strategies have their focal points and confinements. The choice of method depends on the specific characteristics of the redox equation and the level of complexity involved.
Conclusion
The Ion Electron Method and the Oxidation Number Method are two distinct approaches used to balance redox reactions. While the Ion Electron Method focuses on the transfer of electrons between species and employs half-reactions, the Oxidation Number Method assigns oxidation numbers to atoms and determines electron transfer through changes in oxidation numbers. Understanding the key differences between these methods and their respective applications can aid chemists in selecting the appropriate technique for solving redox reactions effectively.
