When we dive into the complexities of cellular apparatus, we experience two imperative components – chaperones and chaperonins. Both these entities are fundamental in ensuring proper protein folding and maintaining cellular function. Despite being related, they exhibit critical differences in their structure, functions, and mechanisms. We are going to set out on a trip to investigate the dissimilarities between chaperones and chaperonins, picking up experiences into their parts and importance inside the cellular world.
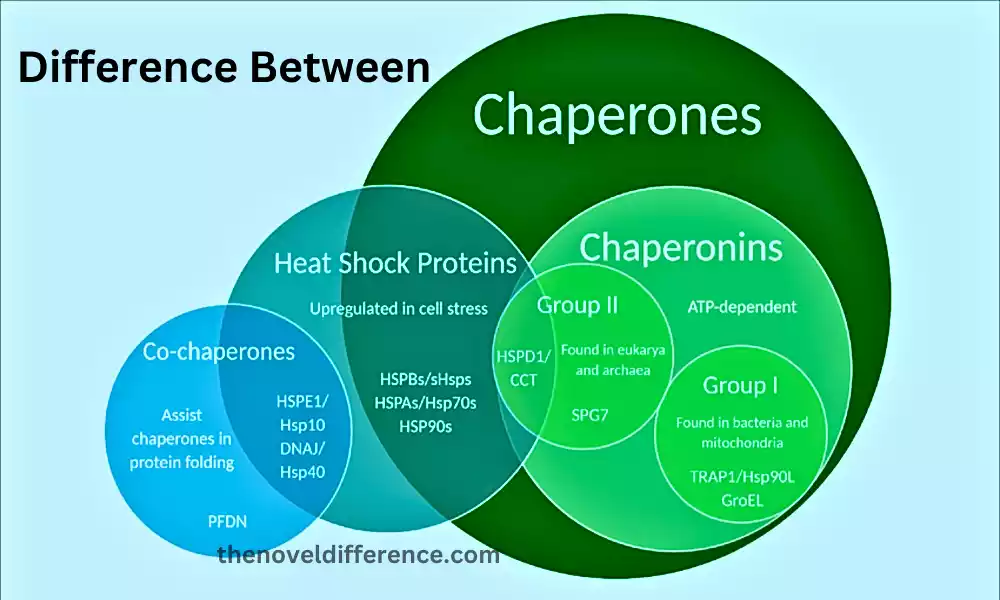
Definition of Chaperones and Chaperonins
Chaperones: Molecular chaperones are a gathering of specialized proteins found in cells that help in the redress and collapse of other proteins. They don’t frame a portion of the ultimate collapsed protein structure, but or maybe transitorily connected with unfurled or mostly collapsed protein substrates, preventing them from accumulating and supporting them in coming to their useful three-dimensional adaptation. Chaperones play a vital part in keeping up protein homeostasis inside the cell and are included in different cellular forms, counting protein amalgamation, debasement, and reaction to stretch. They offer assistance to avoid protein misfolding, which can lead to the arrangement of non-functional or harmful protein totals, involved in a few maladies.
Chaperonins: Chaperonins are a particular lesson of atomic chaperones that have a special and expanded structure, shaping huge round and hollow complexes. These complexes give a sequestered environment for protein collapsing, protected from the cellular environment, and encourage the collapsing of recently synthesized or denatured proteins.
Chaperonins have a place in two fundamental bunches: Bunch I chaperonins, exemplified by GroEL/GroES in microbes, and Bunch II chaperonins, exemplified by TriC/CCT in eukaryotes. Chaperonins work through an ATP-dependent mechanism, where the binding and hydrolysis of ATP lead to conformational changes in the chaperonin complex, allowing for proper protein folding within its central cavity.
Both chaperones and chaperonins play basic parts in protein collapsing, but chaperonins have a more complex structure and give a specialized environment for protein collapsing inside their expansive complexes. Chaperones, on the other hand, interact more transiently with substrates and assist in various cellular processes to ensure proper protein folding and prevent aggregation.
Importance of chaperones and chaperonins in cellular function
The significance of chaperones and chaperonins in cellular work cannot be exaggerated. These atomic players play basic parts in keeping up protein homeostasis and guaranteeing legitimate protein collapsing inside cells. Their functions impact various cellular processes, and their dysregulation can lead to severe consequences, including the development of protein-misfolding diseases.
Here are the key aspects of their significance in cellular function:
1. Protein Folding: The primary role of both chaperones and chaperonins is to assist in protein folding. As proteins are synthesized, they must embrace particular three-dimensional structures to perform their capacities accurately. Chaperones and chaperonins bind to newly synthesized or partially folded proteins, preventing them from misfolding or aggregating prematurely. By providing an environment conducive to folding or guiding the folding process, they ensure that proteins attain their native, functional configurations.
2. Protein Quality Control: Chaperones and chaperonins are integral components of the cellular protein quality control system. They recognize and selectively bind to misfolded or denatured proteins, preventing them from forming harmful aggregates. These misfolded proteins are then either refolded correctly or targeted for degradation, helping to maintain cellular proteostasis.
3. Cellular Stress Response: Oxidative stress, or nutrient deprivation, cells may face an increased load of misfolded or unfolded proteins. Chaperones are upregulated as part of the cellular stress response, assisting in the refolding of damaged proteins and protecting cells from potentially lethal protein aggregation.
4. Protein Degradation Pathways: Chaperones play a role in facilitating the recognition and delivery of misfolded or unwanted proteins to cellular degradation pathways, such as the ubiquitin-proteasome system or autophagy. This guarantees the expulsion of harmed or broken proteins, which is basic for keeping up cellular well-being and avoiding the collection of harmful totals.
5. Regulatory Functions: Chaperones and chaperonins can also have regulatory functions beyond protein folding. Some chaperones interact with specific signaling proteins, modulating their activities or stability. This intelligence can influence cellular signaling pathways, quality expression, and different physiological forms.
6. Protein Transport and Assembly: Chaperonins, with their specialized structures, are involved in the proper folding and assembly of complex protein structures, such as multi-subunit protein complexes or large macromolecular assemblies. They assist in guiding the correct associations between protein subunits and the subsequent formation of functional complexes.
7. Implications in Disease: Dysfunction or imbalances in chaperones and chaperonins have been linked to various diseases, collectively termed protein misfolding diseases. Cases consolidate neurodegenerative clutters like Alzheimer’s, Parkinson’s, and Huntington’s ailment, as well as certain sorts of cancer. Understanding chaperone/chaperonin-related mechanisms in these conditions is critical for potential therapeutic interventions.
Chaperones and chaperonins play indispensable roles in maintaining cellular function by ensuring proper protein folding, quality control, stress response, and protein assembly. Their exercises have significant suggestions for cellular well-being and are closely connected to the advancement and movement of various illnesses.
What are Chaperones?
Chaperones, also known as atomic chaperones, are a gathering of specialized proteins found in cells that play a significant part in protein collapsing and keeping up protein homeostasis. They assist other proteins, known as client proteins, in achieving their correct three-dimensional structure, which is essential for their proper function. Chaperones do not become part of the final folded protein structure; instead, they transiently interact with unfolded or partially folded protein substrates, preventing them from aggregating and guiding them toward their functional conformation.
The process of protein folding is essential for cellular function because proteins need to adopt specific shapes to perform their designated tasks correctly. During various cellular forms, such as protein union, transport, and push reactions, a few proteins may be gotten to be uncovered to unfavorable conditions that can lead to misfolding or fractional unfurling. Misfolded proteins can be non-functional or frame harmful totals, which are related to various illnesses, counting neurodegenerative clutters.
Chaperones act as “molecular bodyguards” for proteins, helping them to fold properly and prevent the formation of non-functional conformations or toxic aggregates. They do so by stabilizing intermediates during the folding process, providing a protected environment for folding, and facilitating the correct interactions between amino acid residues in the protein sequence.
The functions of chaperones are not constrained to protein collapsing; they are also included in other cellular forms, counting protein transport, gathering multi-subunit protein complexes, and controlling protein debasement pathways. Chaperones are a portion of the cellular push reaction, and their expression is frequently upregulated in reaction to natural stressors, making a difference in cells adapting to the expanded stack of misfolded or denatured proteins.
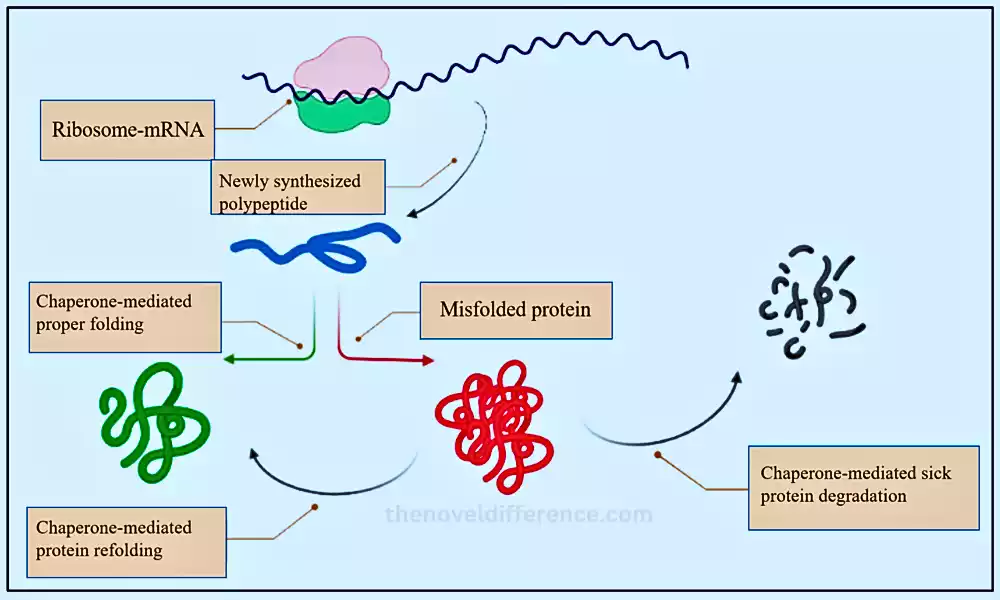
Chaperones play a principal part in keeping up protein homeostasis and guaranteeing that cells work ideally. Their action is basic for appropriate cellular well-being, and their dysregulation can lead to the advancement of protein-misfolding infections and other clutters. Understanding chaperone work and their intuition with client proteins have critical suggestions for different zones of science, counting cell science, atomic science, and medication.
Types of molecular chaperones
Molecular chaperones are a differing gathering of proteins with distinctive structures and capacities. They can be broadly classified into several families based on their primary sequence and modes of action.
Some of the main types of molecular chaperones include:
1. Hsp70 Family: Heat shock protein 70 (Hsp70) is one of the most well-known and extensively studied chaperone families. Hsp70 chaperones assist in the folding of newly synthesized polypeptides and the refolding of denatured proteins. They work in an ATP-dependent manner, using ATP hydrolysis to regulate their interaction with client proteins. The Hsp70 family also includes co-chaperones like Hsp40, which help in substrate recognition and delivery to Hsp70.
2. Hsp90 Family: Heat shock protein 90 (Hsp90) is another essential chaperone family that plays a critical role in the folding and maturation of specific signaling proteins and regulatory factors. Hsp90 chaperones are involved in the stabilization and activation of many client proteins, including various kinases and steroid hormone receptors. Their ATPase activity is essential for their function.
3. Hsp60/Chaperonin Family: This family includes large, barrel-shaped chaperonin complexes, such as GroEL/GroES in bacteria and TriC/CCT in eukaryotes. They provide a specialized environment for protein folding within their central cavity. The Hsp60/Chaperonin family facilitates the folding of a wide range of substrates, including both newly synthesized and denatured proteins.
4. Hsp100 Family: The heat shock protein 100 (Hsp100) family includes chaperones with ATPase activity that are involved in disaggregating and unfolding misfolded or aggregated proteins. They play a crucial role in protein quality control by refolding or targeting damaged proteins for degradation.
5. Small Hsp (sHsp) Family: Small heat shock proteins (sHsps) are a diverse group of chaperones with low molecular weight. They function by binding to unfolding or misfolded proteins, preventing their aggregation and facilitating their refolding. sHsps are found in various cellular compartments and are involved in protecting cells during stress conditions.
6. Prefoldin: Prefoldin is a unique chaperone complex that assists in the folding of newly synthesized proteins by capturing hydrophobic regions of nascent polypeptides, thus preventing inappropriate interactions and assisting in the correct folding process.
7. Hsp110 Family: Hsp110 proteins are nucleotide exchange factors that interact with Hsp70 and assist in the release of bound substrate proteins. They play a role in the refolding of client proteins and participate in protein disaggregation processes.
These are some of the major families of molecular chaperones found in cells. Each family of chaperones performs particular capacities and contributes to the support of cellular protein homeostasis and the avoidance of protein misfolding and conglomeration. Their coordinated activities are essential for ensuring proper protein folding, transport, and cellular function under both normal and stressful conditions.
What are Chaperonins?
Chaperonins are a specific class of molecular chaperones, which are large, multisubunit protein complexes found in cells. They play a crucial role in assisting the correct folding of newly synthesized or denatured proteins in a sequestered environment. Unlike other chaperones that transiently interact with unfolded proteins, chaperonins provide a more controlled and isolated space where protein folding can occur without interference from the cellular environment.
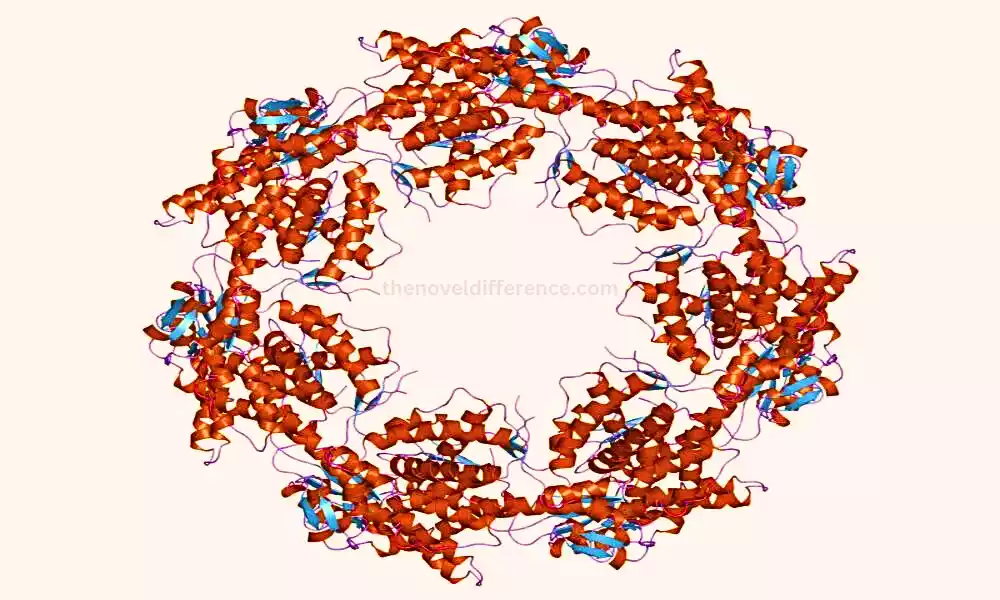
The structure of chaperonins is characterized by a cylindrical or double-ring architecture, formed by multiple subunits arranged in stacked layers. Each chaperonin complex has an inner cavity that provides a protected folding chamber for unfolded or misfolded proteins. The collapsing preparation inside this chamber is ATP-dependent, meaning it requires vitality within the frame of ATP hydrolysis to encourage conformational changes and help in protein collapsing.
There are two main groups of chaperonins:
1. Group I Chaperonins: This group includes bacterial chaperonins, such as GroEL (Hsp60) and its co-chaperonin GroES. GroEL is composed of two back-to-back rings, each made up of seven indistinguishable subunits, coming about a add up to 14 subunits. The GroES co-chaperonin forms a lid-like structure that caps one end of the GroEL complex. Together, GroEL and GroES create a folding chamber where protein substrates are encapsulated for proper folding.
2. Group II Chaperonins: Group II chaperonins are found in eukaryotes and archaea. A well-known example is TriC (also known as CCT – chaperonin-containing TCP-1), which is composed of eight different subunits arranged in two stacked rings, with eight subunits in each ring.
The chaperonin-assisted protein folding process involves several steps:
1. Binding: The unfolded or partially folded protein substrate binds to the central cavity of the chaperonin complex.
2. Encapsulation: The chaperonin complex encapsulates the protein substrate within its folding chamber, shielding it from the cellular environment.
3. Conformational Changes: ATP hydrolysis induces conformational changes in the chaperonin complex, leading to the closure of the folding chamber. This step facilitates the proper folding of the substrate protein.
4. Release: Upon successful folding, the chaperonin complex opens up, and the correctly folded protein is released into the cellular environment.
Chaperonins are fundamental for the proper collapsing of a wide run of proteins, counting those included in crucial cellular forms such as protein transport, flag transduction, and cytoskeletal get-together. Their inclusion in helping protein collapse and anticipating misfolding is basic for cellular homeostasis and anticipating the arrangement of protein totals that can lead to different illnesses.
Mechanism of action
The mechanism of action of chaperonins involves an intricate process that relies on ATP-dependent conformational changes to assist in protein folding within their sequestered chambers.
The primary steps in the mechanism are as follows:
1. Binding of the Unfolded Protein: The first step involves the binding of the unfolded or partially folded protein substrate to the chaperonin complex. The substrate interacts with specific binding sites within the central cavity of the chaperonin, ensuring proper encapsulation.
2. Encapsulation of the Substrate: Once the substrate is bound, the chaperonin complex undergoes an ATP-dependent conformational change, which leads to the closure of its folding chamber. In the case of Group I chaperonins (e.g., GroEL/GroES), the co-chaperonin GroES forms a “lid” that caps one end of the GroEL complex, effectively sealing the chamber and isolating the substrate protein from the surrounding cellular environment.
3. ATP Hydrolysis and Conformational Changes: ATP hydrolysis is a critical step in the chaperonin mechanism. ATP molecules bound to the chaperonin subunits are hydrolyzed to ADP and inorganic phosphate (Pi). This hydrolysis process induces conformational changes in the chaperonin complex, which facilitates the proper folding of the substrate protein within the confined space of the folding chamber.
4. Substrate Folding and Release: As a consequence of ATP hydrolysis, the chaperonin complex undergoes conformational changes, which leads to the release of the substrate protein in a folded or partially folded state. The chaperonin complex can then be reloaded with another substrate protein, starting the cycle anew.
The process of chaperonin-assisted protein folding is highly regulated and requires precise coordination between ATP binding, hydrolysis, and substrate binding and release. The binding and encapsulation of the substrate protein within the chaperonin chamber protect it from premature interactions with other cellular components, allowing it to fold correctly without interference. The ATP-dependent conformational changes provide the necessary energy to facilitate the folding process and ensure that the substrate is released in a properly folded state.
The ATP-driven conformational changes in chaperonins create a specialized environment that promotes efficient and correct protein folding, preventing the formation of misfolded or aggregated proteins. This component is vital for keeping up protein homeostasis inside cells and plays a crucial part in different cellular forms and infection avoidance.
Difference Between Chaperones and Chaperonins
Chaperones and chaperonins are both sorts of atomic chaperones, but they have particular characteristics and components of activity.
Here are the key differences between chaperones and chaperonins:
1. Structure:
• Chaperones: Molecular chaperones are typically smaller, single polypeptide chain proteins that assist in protein folding. They do not have a well-defined structural motif and can interact transiently with unfolded or partially folded protein substrates.
• Chaperonins: Chaperonins are larger, multisubunit protein complexes with a characteristic cylindrical or double-ring architecture. They have a more elaborate structure, with multiple subunits arranged in stacked rings, forming a folding chamber where proteins can fold in a sequestered environment.
2. Mode of Action:
• Chaperones: Chaperones interact with unfolded or partially folded protein substrates in an ATP-independent manner. They help stabilize intermediates during the folding process, prevent aggregation, and guide the substrate toward its functional conformation.
• Chaperonins: Chaperonins facilitate protein folding through an ATP-dependent mechanism. ATP hydrolysis induces conformational changes in the chaperonin complex, leading to the encapsulation of the substrate protein within the folding chamber. The chaperonin chamber provides a controlled environment for the substrate to fold correctly, shielded from the cellular environment.
3. Substrate Binding:
• Chaperones: Chaperones bind to unfolded or partially folded proteins through hydrophobic interactions and other non-covalent forces. They transiently associate with the substrate and release it once the folding process is complete.
• Chaperonins: Chaperonins bind to substrate proteins in a more complex manner. The substrate is first captured by the chaperonin, and then ATP hydrolysis induces conformational changes that lead to the full encapsulation of the substrate within the folding chamber.
4. Function:
• Chaperones: Molecular chaperones assist in the folding, refolding, and stabilization of a wide range of proteins. They play basic parts in different cellular forms, counting protein blend, transport, corruption, and cellular push reaction.
• Chaperonins: Chaperonins are particularly involved in assisting the folding of larger, more complex, or slow-folding proteins. They are significant for the proper collapsing of numerous fundamental proteins, such as those included in flag transduction, cytoskeletal get-together, and protein transport.
5. Examples:
• Chaperones: Examples of chaperones include the Hsp70 and Hsp90 families, which are single polypeptide chain proteins that bind to unfolded or partially folded substrates.
• Chaperonins: Examples of chaperonins include GroEL/GroES in bacteria (Group I chaperonins) and TriC/CCT in eukaryotes (Group II chaperonins).
Chaperones and chaperonins are both vital for protein collapsing, but they contrast in estimate, structure, mode of activity, and the sort of substrates they help in collapsing. Chaperones have a more general role and interact transiently with substrates, while chaperonins provide a specialized environment for the folding of specific, larger, or more complex proteins in an ATP-dependent manner.
Similarities Between Chaperones and Chaperonins
Whereas chaperones and chaperonins have particular characteristics and instruments of activity, they moreover share a few similitudes in their parts and capacities as atomic chaperones.
Here are some of the key similarities between chaperones and chaperonins:
1. Protein Folding Assistance: Both chaperones and chaperonins play essential roles in assisting the correct folding of proteins. They offer assistance in anticipating protein misfolding, conglomeration, and the arrangement of non-functional conformations, which can be destructive to cellular work.
2. Protein Quality Control: Chaperones and chaperonins are involved in the cellular protein quality control system. They recognize and selectively bind to misfolded or denatured proteins, preventing their accumulation and promoting either their refolding or targeting for degradation.
3. Cellular Stress Response: Both chaperones and chaperonins are part of the cellular stress response. When cells are exposed to natural stresses like warm stun or oxidative stretch, the expression of chaperones and chaperonins is frequently upregulated to assist in overseeing the expanded stack of unfurled or denatured proteins.
4. ATP-Dependent Mechanism: While the ATP-dependent mechanism is more characteristic of chaperonins, some chaperones, such as members of the Hsp70 family, also utilize ATP to regulate their chaperone activity. ATP official and hydrolysis play a significant part in the energetic intuition between chaperones and their substrate proteins.
5. Involvement in Protein Transport: Both chaperones and chaperonins are involved in facilitating the transport of proteins to their proper cellular locations. Chaperones can assist in guiding nascent polypeptides to specific cellular compartments, while chaperonins may assist in the folding of proteins destined for complex assemblies or specific organelles.
6. Preventing Protein Aggregation: Both types of molecular chaperones prevent the aggregation of unfolded or misfolded proteins. By binding to exposed hydrophobic regions of the substrate proteins, they shield them from non-specific interactions and promote correct folding.
7. Roles in Disease: Both chaperones and chaperonins have implications for various diseases. Dysregulation or changes in chaperones or chaperonins have been related to protein misfolding sicknesses and neurodegenerative clutters, such as Alzheimer’s, Parkinson’s, and Huntington’s illness.
8. Cooperative Interactions: Chaperones and chaperonins can work together cooperatively in protein folding pathways. Some chaperones may deliver partially folded substrates to chaperonins for further assistance in achieving their native conformation.
Chaperones and chaperonins share several fundamental functions related to protein folding and quality control, and both are vital for maintaining cellular proteostasis. While they have distinct mechanisms and structures, their cooperative interactions and overlapping roles contribute to the efficient management of protein folding processes within cells.
Conclusion
The difference between chaperones and chaperonins lies in their mechanism of action, size, scope of assistance, refolding roles, and catalytic nature. While both contribute significantly to cellular health and function, they play distinct roles in ensuring the proper folding of proteins. Their strengths, when combined, provide a formidable defense against cellular proteotoxic stress. Understanding these two essential cellular entities deepens our appreciation for the intricate world of protein folding and quality control.