Introduction of Fullerene and Carbon Nanotubes
Carbon, a plethora of common elements, is the base of life as well as a myriad of substances. In the vast variety of carbon-based structures, fullerenes as well as carbon nanotubes have attracted a lot of attention because of their distinctive chemical and physical properties. Both are carbon allotropes, and their similarities and differences are often the focus of interest to scientists.
Definition of Carbon Allotropes
Allotropes are various structural modifications of elements where elements are joined in various ways. When it comes to carbon, it’s found in three natural allotropes such as graphite, diamond, and Amorphous carbon. In the past, scientists have created additional carbon allotropes like carbon nanotubes and fullerenes.
Brief Overview of Carbon’s Ability to Form Various Structures
Carbon is an extraordinary element of the periodic table principally because of its capacity to create a variety of forms. This ability is due to the carbon’s distinctive electronic arrangement with four electrons that are valence. Carbon can create the four bonds that covalently bond other elements, which is why it has a plethora of bonding abilities.
Below is a quick description of the power of carbon in the formation of different structures:
- Diamond: With this type of carbon atoms are connected tetrahedrally to four carbon atoms, creating a three-dimensional structure of the lattice. This covalent network provides diamond with its remarkable hardness which makes it one of the most durable natural substances. Diamond is also a great conductor of heat as well as an electrical conductor and insulator.
- graphite: Carbon atoms here are joined in two-dimensional, flat hexagonal rings that are arranged in two-dimensional planes. Carbon atoms form three sigma bonds to three carbons that are adjacent, and the fourth valence electron creates pi bonds, which are which is delocalized across all of the planes. This delocalization provides graphite with conductivity as a metal. The layers on these plans are joined through van der Waals force permitting them to slide over each other, giving graphite the characteristic slippy feel.
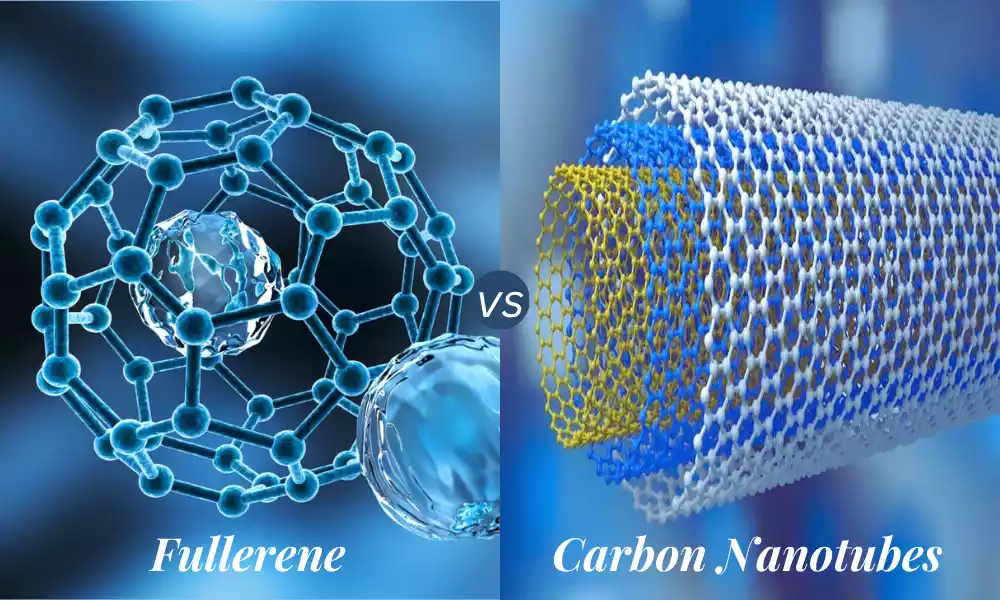
- Fullerenes: The fullerenes are the spherical, ellipsoidal, or tubular structures composed of carbon. The most well-known fullerene is the C60 chemical, which is also known as the “buckyball”, which resembles a soccer ball comprised of hexagons and pentagons. Fullerenes come in a variety of sizes but they have an open cage-like structure.
- Carbon Nanotubes (CNTs): They are cylindrical structures made out of rolled graphene sheets. They are able to have a single wall (with only one concentric single layer) as well as multiwalled (with many layers of concentric). CNTs have remarkable thermal, mechanical, as well as electrical properties.
- graphene: One layer composed of carbon atoms, arranged into an intricate honeycomb structure in two dimensions. It is a marvel of electronic conductivity, flexibility, and strength. It is considered to be a fundamental material for future electronics as well as other applications.
- Amorphous Carbon: It is a kind of carbon that carbon atoms do not have any crystalline order of long-range. Examples include soot and coal. It may contain a mix of structures, similar to those in diamond and graphite along with different carbon-based allotropes.
- Carbon Nanofoam: A recent discovery, it is a three-dimensional, low-density network of carbon atoms that are bonded in a fractious manner some of them making flat graphene-like shapes while others form polyhedral forms.
- Lonsdaleite (Hexagonal Diamond): Formed when meteorites with graphite hit the Earth this carbon allotrope has a hexagonal arrangement of crystalline. It is much harder than normal diamonds in certain directions.
The unbeatable versatility of carbon in forming different structures is evidence of its unique bonding and electronic properties. Every carbon allotrope has distinctive properties that are useful for a variety of uses, ranging from jewelry (diamonds) to electronic devices (graphene) and structural materials (carbon nanotubes).
What is Fullerene?
Fullerenes, also known as ‘buckyballs or ‘buckytubes’ are carbon atoms that are formed into hollow spheres, hollow ellipsoids tubes, or any other shape. The most well-known fullerene type is the C60 which resembles a soccer ball that is made up of 12 pentagons and 20 hexagons with the carbon atom located at every vertex.

History:
Fullerenes are named after Richard Buckminster Fuller, an architect renowned for his Geodesic Dome style and a close resemblance to the C60 structure of the molecule. They were first identified in 1985 by the researchers who included Sir Harold Kroto, and Robert Curl along with Richard Smalley, who later received the Nobel Prize in Chemistry for their pioneering research.
Properties and Uses:
- Physical Properties: Fullerenes exhibit a dark, soot-like appearance. They’re soluble in a variety of organic solvents.
- Chemical properties: Fullerenes can undergo diverse reactions, making them useful for chemical modifications. For instance, they could serve as electron acceptors and lead to the creation of negatively charged negatively charged ions.
- applications: Fullerenes have potential applications in the field of medicine (e.g. drugs, for example) and in the field of materials sciences (as superconductors when infused by certain elements) as well as in electronics (as organic semiconductors).
What are Carbon Nanotubes (CNTs)?
Carbon nanotubes can be described as cylindrical structures comprised of a rolled-up sheet made of a single-layer carbon lattice known as graphene. Based on the number of layers they could be classified as single-walled carbon Nanotubes (SWCNTs) and multi-walled carbon Nanotubes (MWCNTs).

History:
Although the idea of carbon nanotubes is dated from the beginning of the 20th century however it wasn’t until the 1990s that they began to receive much attention. Sumio Iijima, a Japanese scientist, is usually acknowledged as the one who discovered them during his research into the material residues that remained from an arc-discharge device.
Properties and Uses:
- Physical Property: CNTs are known for their remarkable thermal conductivity and tensile force. They also exhibit quantum mechanical properties on the nanoscale.
- Chemical properties: Carbon nanotubes may be functionalized. This means they can bind to other kinds of molecules or atoms on their surface, changing their properties.
- Application: CNTs are investigated for their use in a vast variety of applications ranging that span nanotechnology, electronics, and optics, to the creation of novel materials that have unique properties. The potential applications include the delivery of biomedicine drugs as fillers for composite materials and for the creation of small and ultra-fast electronic components.
Fullerene and Carbon Nanotubes in the Comparison Chart
| Feature | Fullerene | Carbon Nanotubes |
|---|---|---|
| Basic Structure | Spherical or ellipsoid | Cylindrical |
| Common Types | C60, C70, etc. | SWCNTs, MWCNTs |
| Discovery Year | 1985 | The early 1990s |
| Main Components | Carbon | Carbon |
| Bonding Pattern | Hexagons and Pentagons | Hexagons |
| Electrical Properties | Semiconductor | Can be metallic or semi-conductive |
| Physical Appearance | Dark, soot-like | Black or dark grey tubes |
| Applications | Medicine, Electronics, etc. | Nanotechnology, Electronics, etc. |
What are the Similarities between Fullerene and Carbon Nanotubes?
Carbon nanotubes and Fullerenes both carbon nanotubes, which are allotropes are both composed of carbon and share a variety of striking similarities that are rooted in their composition, structure, and properties.
These are the most important similarities between them:
- Carbon Composition: Fullerenes, as well as carbon nanotubes, are completely made of carbon atoms.
- Molecular arrangement: The carbon atoms in both structures are placed in hexagonal shapes. Fullerenes also include pentagonal (or sometimes Heptagonal) configurations to form circular or ellipsoidal structures Carbon nanotubes are in essence extended tubes with hexagonal arrangement.
- Origins from Graphene: If you think of graphene to be a flat, one-atom-thick layer of carbon atoms placed in hexagons, both carbon nanotubes and fullerenes are able to be visualized through the manipulation of this sheet. Fullerenes can be viewed as graphene sheets that are wrapped into spheres. Carbon nanotubes are pictured as graphene sheets folded into cylindrical shapes.
- Synthesis methods: Fullerenes as well as CNTs are synthesized with similar methods, like laser ablation, arc discharge, and chemical deposition of vapor (CVD).
- Conductivity in Electrical Circuits: Both fullerenes and certain carbon nanotubes (specifically the single-walled carbon nanotubes (SWCNTs) are able to display semiconducting properties. It is important to be aware that certain carbon nanotubes may also display metallic properties, based on the chiral direction they’re in.
- Reactivity: The two materials were identified as chemically reactive, particularly when functionalized or if defects are present. This enables modification of them to meet various needs, including drug delivery, to composite development of materials.
- Application within Nanotechnology: Both fullerenes and carbon nanotubes are at the leading edge of nanotechnology research because of their distinct characteristics at the nanoscale. This leads to applications in medicine, electronics as well as materials science, and much more.
- The potential for drug delivery: Fullerenes as well as carbon nanotubes are under investigation for applications in drug delivery. Their capability to be functionalized is a good reason to use them for attaching drugs to particular sites in the body.
- High Surface-to-Volume Ratios: This property, which is present in many nanomaterials, enables the interaction of a vast amount of molecules or atoms on their surfaces, making them suitable for applications such as catalysis or Adsorption.
- Quantum Effects: Both materials show quantum effects because of their nanoscale size which is a subject of intensive research, particularly in quantum computing and electronics applications.
Although carbon nanotubes and fullerenes possess distinct structures and characteristics that distinguish them they have a number of fundamental similarities because of their carbon-based structure and hexagonal molecular arrangement. The shared properties and features are at the core of numerous inventive applications across a variety of areas.
Applications in Modern Technology and Research
Fullerenes:
- Drug Delivery: Their hollow interior and ability to undergo functionalization make them suitable for drug delivery applications.
- Photovoltaics: They can act as electron acceptors in organic solar cells.
- Superconductors: Doped fullerenes show superconductivity.
Carbon Nanotubes:
- Electronics: Potential to replace silicon in certain semiconductor applications.
- Composite Materials: Enhancing the strength and conductivity of polymers.
- Sensors: Can detect gases, chemicals, and even DNA.
Environmental and Health Impacts
Environmental and Health Impacts of Fullerene and Carbon Nanotubes
As a result of the boom in nanotechnology, there is an increasing curiosity about the health and environmental impacts of nanomaterials such as fullerenes and carbon nanotubes (CNTs). While these materials are promising applications across various sectors, there are worries about their potential impacts on human health as well as the environment.
- Environmental Impact:
Fullerenes:
- water systems: Fullerenes may accumulate within water systems, affecting microorganisms, and possibly becoming part of the food chain. Due to their hydrophobic nature, they are prone to aggregation and can pose dangers to aquatic life.
- Soil Interaction: The relationship of fullerenes with soil constituents remains under study. Initial studies suggest that they could alter the growth of specific species of plants and soil microbes.
Carbon Nanotubes:
- System of Water: Similar to fullerenes CNTs also can be found within water bodies. Their fibrous, long structure could pose a problem for aquatic organisms.
- Soil Interaction: CNTs may hinder the growth of some plants by affecting the uptake of nutrients or altering the growth of roots.
- Health Impacts:
Fullerenes:
- Toxicity: The initial studies suggested that fullerenes derivatized by certain chemicals may cause oxidative stress within cells, which could lead to the development of cytotoxic effects.
- Respiratory Issues: Inhalation of fullerene particles can cause respiratory problems, but the exact risk is being investigated.
Carbon Nanotubes:
- Toxicity: The toxicity of CNTs is influenced by a variety of variables, including length, the degree of aggregation, and surface modification. Certain CNTs may trigger inflammatory and fibrotic reactions when they are introduced to the lungs of animals.
- Respiratory Risks: Because of its fibrous character, there is a risk that CNTs may have similar risks to respiratory health like asbestos if inhaled. Straight, long CNTs particularly are known to be able to induce asbestos-like pathogenicity through preliminary research.
- Permeation of Skin: There’s limited evidence showing that CNTs are able to penetrate skin that is intact however they could be a risk when they come into contact with damaged skin, or by occupational exposures.
Mitigation and Current Practices:
As the awareness of potential health and environmental impacts increases, researchers and industries are seeking safe methods to produce, handle as well and dispose of.
- Containment: Utilizing protective barriers and strategies for containment during processing and manufacturing can reduce the release of pollutants into the environment.
- Personal Protective Equipment (PPE): workers in industries that make or utilize these materials are typically mandated to use PPE including gloves, masks, and other protective gear.
- Waste Management: The proper disposal techniques which include recycling opportunities or the containment of waste materials can reduce exposure to the environment.
- Studies: Continued research is essential to understand the long-term health and environmental consequences of CNTs and fullerenes. This research will aid in guiding rules and safety procedures in the near future.
Conclusion
Carbon nanotubes and fullerenes which both originate from carbon’s many uses, have unique properties that are pushing the limits of electronic engineering, materials science, and medicine. But, as they expand in use knowing their impact on health and the environment is crucial.