Calcination and sintering are two distinct processes that play crucial roles in shaping and transforming various substances. Although they share similarities and are often used in conjunction, it is essential to understand the key differences between calcination and sintering to fully comprehend their significance and applications. This article seeks to delve deeper into both processes, exploring their fundamentals as well as methods and results.
Definition of Calcination and Sintering
Calcination: Calcination is a treatment process in which materials are heated at extreme temperatures of 700-1000 degree Celsius or greater in an oxygen or air-free environment, typically between 700-1100 degree Celsius (or greater) without oxygen present or with only limited air present. When this procedure takes place there will be physical and chemical transformations taking place within the material; eliminating volatile substances while decomposing certain components and altering some physical properties of it as a result of heating at such high levels. The process is often used to drive off water, carbon dioxide, and other volatile impurities, leaving behind a more refined or purified material.
Sintering: Sintering is a process of compacting and forming solid materials into a coherent mass using heat and pressure. Heating ceramic or metal powders to temperatures lower than their melting points is known as pelletizing. The elevated temperature causes the particles to bond together through various mechanisms, including diffusion, plastic deformation, and solid-state reactions. Sintering leads to the formation of a densified material with improved mechanical strength, reduced porosity, and enhanced structural integrity. Ceramic, metal parts, and composite material production is done using this system.
Importance of understanding the difference between calcination and sintering
Understanding the difference between calcination and sintering is important for several reasons:
1. Process Optimization: Calcination and sintering are distinct processes with different temperature ranges, chemical reactions, and outcomes. Understanding their differences allows for proper process optimization. By selecting the appropriate process for a given material, one can achieve the desired results efficiently and effectively.
2. Material Selection: Different materials respond differently to calcination and sintering processes. Knowing the distinction helps in selecting the suitable process for a specific material to achieve the desired properties, such as purity, strength, density, or porosity. Information such as this is especially vital to industries including metallurgy, ceramics, and construction materials.
3. Quality Control: Understanding the differences between calcination and sintering is essential for quality control during manufacturing processes. By monitoring and controlling the parameters specific to each process, such as temperature, time, and atmosphere, manufacturers can ensure consistent and reliable product quality.
4. Product Development: Such as catalysts, semiconductors, and high-performance ceramics, understanding calcination and sintering enables researchers to explore and develop new materials with tailored properties. By manipulating the processes, they can optimize the microstructure, composition, and functionality of the final product.
5. Troubleshooting and Problem Solving: Differentiating between calcination and sintering is valuable in troubleshooting issues that may arise during manufacturing. If there are deviations in product quality or undesired changes in material properties, understanding the processes involved helps identify potential causes and implement corrective actions more effectively.
6. Innovation and Process Improvement: Understanding the differences between calcination and sintering can inspire innovation and process improvement. Researchers and engineers can explore modifications, alternative techniques, or combinations of these processes to achieve new or enhanced material properties, leading to advancements in various industries.
Comprehending the distinction between calcination and sintering allows for informed decision-making, efficient manufacturing processes, improved product quality, and the potential for innovation and advancement in materials science and related fields.
What is Calcination?
Calcination involves heating a material to temperatures exceeding 700-1000 Celsius when there is either no oxygen supply or very limited access. This process is primarily used to drive off volatile components, decompose certain compounds, and alter the physical and chemical properties of the material.
During calcination, various chemical and physical changes occur within the material. Common transformations involve dehydration and carbonation processes which release carbon dioxide as waste products; reduction or oxidation of specific elements and the breaking down of organic material or unstable compounds are other processes commonly undertaken during transformations.
The specific purpose of calcination depends on the nature of the material being treated. It can serve to purify the substance by removing impurities, improving its thermal stability, enhancing its reactivity, or changing its phase or crystal structure.
Calcination is widely applied in numerous industries. For example, in the production of quicklime, limestone is subjected to calcination to drive off carbon dioxide and convert it into calcium oxide, which is a key component in cement production. Similarly, calcination is used in the roasting of ores in metallurgy to remove volatile impurities and convert metal sulfides into metal oxides.
Calcination is a crucial thermal treatment process that plays a significant role in various industries, enabling the refinement, purification, and transformation of materials through the application of high temperatures in controlled environments.
Key characteristics of calcination
Calcination possesses several key characteristics that distinguish it as a specific thermal treatment process.
These characteristics include:
1. Temperature Range: Calcination typically occurs at high temperatures, typically in the range of 700 to 1000 degrees Celsius (or higher), depending on the material being processed. The temperature range is often determined by the specific reactions and transformations required for the material.
2. Chemical Reactions: Calcination involves a variety of chemical reactions within the material. These reactions can be described by the removal of volatile substances (like carbon dioxide ) as well as decomposition of specific materials; reduction or oxidation of elements; and transformations from unstable to more stable compounds.
3. Physical Changes: The process of calcination induces physical changes in the material. These changes can include shrinkage or reduction in volume, as volatile components are driven off, and the formation of new phases or crystal structures due to the chemical transformations taking place.
4. Controlled Atmosphere: Calcination is often carried out in the absence or limited supply of air or oxygen. This controlled atmosphere prevents unwanted reactions, such as combustion or oxidation, and ensures the desired chemical transformations occur.
5. Time and Heating Rate: The duration of calcination varies depending on the material and desired outcomes. It can range from several minutes to several hours or even longer. The heating rate, or the rate at which the temperature is increased, is also an important factor to consider for optimal calcination results.
6. Equipment and Furnace Design: Calcination is typically conducted in specialized equipment and furnaces capable of reaching and maintaining high temperatures. The design of this equipment and furnaces ensures uniform heating and control over the process parameters, such as temperature, atmosphere, and residence time.
Understanding these key characteristics of calcination is crucial for effectively implementing the process in various industries. By controlling the temperature, atmosphere, and other process parameters, manufacturers can achieve the desired chemical and physical transformations in materials, leading to improved product quality, purity, stability, and functionality.
Temperature range
The temperature range for calcination typically falls within the range of 700 to 1000 degrees Celsius (or higher), although the specific temperature required can vary depending on the material being processed and the desired transformations.
Temperature variations depend upon various factors such as the chemical composition of materials present and any reactions or chemical processes taking place as well as the thermal stability of substances present. Different materials have different temperature thresholds for undergoing calcination.
Calcination may require temperatures above 1000 degrees Celsius, especially for materials that have high melting points or compounds that require significant energy input for decomposition. For example, in the calcination of certain refractory materials or mineral ores, temperatures above 1200 degrees Celsius may be necessary.
It is important to note that the temperature range for calcination is distinct from other thermal treatment processes such as sintering or melting. Calcination is typically carried out at temperatures below the melting point of the material, ensuring that only chemical and physical transformations occur without complete liquefaction.
The selection of the appropriate temperature range for calcination is crucial to achieving the desired outcomes, such as the removal of volatile components, decomposition of compounds, or phase transitions. Controlling the temperature within the specified range is vital for efficient and effective calcination processes in various industries, including cement production, metallurgy, ceramics, and minerals processing.
Chemical reactions involved
Calcination involves various chemical reactions depending on the composition of the material being processed.
The specific reactions occurring during calcination can vary, but here are some common chemical reactions that may take place:
1. Dehydration: Many materials contain water molecules or hydroxyl groups that can be removed during calcination. The chemical reaction involved is typically dehydration, where water molecules are eliminated. For example, the calcination of gypsum (calcium sulfate dihydrate) involves the removal of water to form calcium sulfate hemihydrate (plaster of Paris).
CaSO4 · 2H2O → CaSO4 · 0.5H2O + 1.5H2O(g)
2. Decarbonisation: Calcination can also involve the release of carbon dioxide from carbonate minerals. This reaction is called decarbonization. For instance, the calcination of limestone (calcium carbonate) leads to the formation of calcium oxide (quicklime) and carbon dioxide.
CaCO3 → CaO + CO2(g)
3. Decomposition: Some compounds undergo decomposition during calcination, resulting in the formation of new compounds. For example, during the calcination of certain metal hydroxides, such as zinc hydroxide, water is eliminated, and the metal oxide is formed.
Zn(OH)2 → ZnO + H2O(g)
4. Oxidation and Reduction: Depending on the material, calcination can involve oxidation or reduction reactions. In the presence of an oxidizing or reducing atmosphere, certain elements within the material can undergo chemical transformations. This can lead to the formation of new compounds or changes in the oxidation states of elements.
These are just a few examples of chemical reactions that can occur during calcination. The specific reactions depend on the composition of the material, the presence of impurities, and the desired transformations. Understanding these chemical reactions is crucial for controlling and optimizing the calcination process to achieve the desired outcomes, such as the removal of volatile components, purification, or phase transitions.
What is Sintering?
Sintering is a process of compacting and forming solid materials into a coherent mass using heat and pressure. It involves heating a powdered or granulated material, such as metal powders or ceramics, to a temperature below its melting point. During sintering, the material particles bond together, resulting in the formation of a densified solid with improved mechanical strength, reduced porosity, and enhanced structural integrity.
The sintering process involves several key steps:
1. Particle Packing: The starting material is typically in the form of fine powders or granules. These particles are carefully engineered to achieve optimal packing density, ensuring efficient particle-to-particle contact during sintering.
2. Heating: The compacted or shaped material is subjected to elevated temperatures below its melting point. As the temperature increases, the particles start to interact and undergo physical and chemical changes.
3. Particle Bonding: At elevated temperatures, the particles experience a diffusion of atoms or ions. This diffusion allows for atomic rearrangement and the formation of bonds between adjacent particles. The bonding mechanisms can include solid-state diffusion, surface diffusion, plastic deformation, and even some degree of melting at particle contact points.
4. Densification: As the particles bond together, the material undergoes densification. This leads to a reduction in porosity and an increase in the density of the sintered mass. The degree of densification depends on factors such as temperature, time, and applied pressure, if any.
5. Grain Growth: During sintering, the individual particles may grow in size as a result of the rearrangement and bonding processes. This grain growth contributes to the strengthening of the sintered material.
The specific parameters of the sintering process, such as temperature, time, pressure, and atmosphere, are carefully controlled to achieve the desired material properties. These properties can include enhanced mechanical strength, improved electrical conductivity, increased thermal stability, and tailored microstructures.
Sintering is widely used in various industries. It is commonly employed in the manufacturing of metal Such as automotive parts, bearings, and cutting tools. Additionally, sintering is utilized in the production of ceramic products, such as tiles, bricks, and advanced ceramics for electronic applications. The process also finds application in the creation of composite materials, where different materials are sintered together to form a unified structure with unique properties.
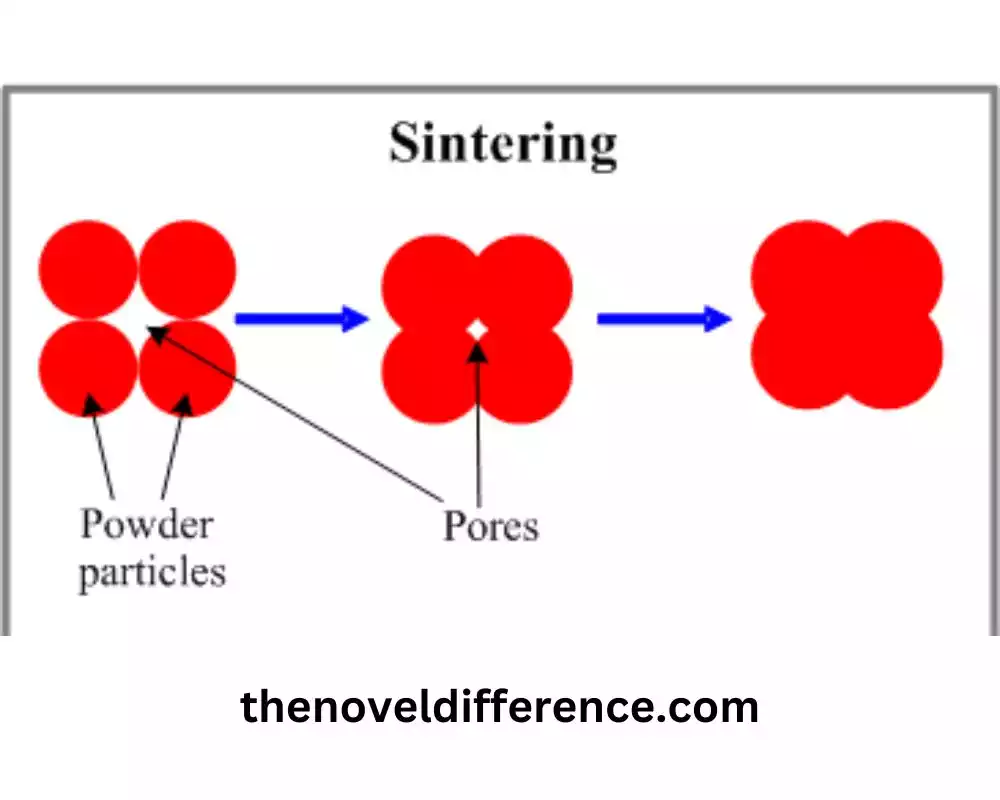
Sintering is a vital process for achieving densification, bonding, and material consolidation, resulting in improved properties and the formation of solid materials with enhanced structural integrity and performance.
Key characteristics of sintering
Sintering possesses several key characteristics that distinguish it as a specific material processing technique.
These characteristics include:
1. Temperature Range: Sintering occurs at temperatures below the melting point of the material being processed. It typically takes place in a temperature range where the material particles exhibit atomic diffusion and bonding but do not fully liquefy. The specific temperature range depends on the material composition and desired outcomes.
2. Particle Bonding Mechanisms: Sintering involves the bonding of individual particles to form a cohesive mass. This bonding can occur through various mechanisms, including solid-state diffusion, surface diffusion, plastic deformation, and even localized melting at particle contact points. These bonding mechanisms lead to the formation of necks or bridges between particles, resulting in increased structural integrity.
3. Densification: Sintering leads to the densification of the material. As the particles bond together, the void spaces between them decrease, resulting in reduced porosity and increased density. Densification is crucial for improving the mechanical strength and other physical properties of the sintered material.
4. Grain Growth: During sintering, the individual particles may undergo grain growth, where they increase in size due to atomic rearrangement and particle coalescence. Grain growth contributes to the strengthening of the material and can influence its microstructure, affecting properties such as hardness, toughness, and grain boundary characteristics.
5. Time and Pressure: The duration of the sintering process varies depending on the material and desired outcomes. The time required for sintering depends on factors such as the material composition, particle size, temperature, and applied pressure (if any). Pressure-assisted sintering techniques, such as hot pressing or spark plasma sintering, can enhance densification and reduce sintering times.
6. Atmosphere and Environment: The sintering process can take place under various atmospheric conditions, including vacuum, inert gas, or controlled gas environments. The choice of the atmosphere depends on the material properties and reactions involved. In some cases, specific atmospheres may be necessary to prevent oxidation, control chemical reactions, or modify the surface chemistry of the sintered material.
Understanding these key characteristics of sintering is essential for optimizing the process parameters and achieving the desired material properties. By controlling factors such as temperature, time, pressure, atmosphere, and particle characteristics, manufacturers can tailor the sintering process to meet specific requirements in industries such as metallurgy, ceramics, powder metallurgy, and advanced materials manufacturing.
Temperature range
The temperature range for sintering typically varies depending on the materials being processed and their specific properties. However, sintering generally occurs at temperatures below the melting point of the material.
The temperature range for sintering can be categorized as follows:
1. Low-Temperature Sintering: This range typically falls between 500 to 800 degrees Celsius (932 to 1472 degrees Fahrenheit). It is commonly associated with materials such as certain ceramics, glass, and certain metal alloys that have low melting points. In this range, particle diffusion and bonding occur, enabling densification and structural formation.
2. Intermediate-Temperature Sintering: This range typically falls between 800 to 1200 degrees Celsius (1472 to 2192 degrees Fahrenheit). It is often used for materials like advanced ceramics, ceramic-metal composites, and certain metal alloys. In this range, enhanced atomic diffusion and grain growth contribute to densification and structural development.
3. High-Temperature Sintering: This range typically exceeds 1200 degrees Celsius (2192 degrees Fahrenheit) and can extend to temperatures as high as 1600 degrees Celsius (2912 degrees Fahrenheit) or more. It is typically employed for materials such as refractory ceramics, superalloys, and certain metal powders. In this range, significant atomic diffusion and grain growth occur, leading to high densification and the development of desired material properties.
The selection of the appropriate temperature range for sintering is critical for achieving the desired outcomes, such as densification, bonding, and structural formation, while avoiding excessive melting or deformation. It is important to consider factors such as material composition, particle size and distribution, desired properties, and the limitations of the materials and equipment being used.
It is worth noting that the temperature ranges provided here are general guidelines and can vary depending on the specific materials and applications involved in the sintering process. The optimization of temperature parameters is crucial to achieving the desired material properties and ensuring the successful fabrication of sintered products in various industries.
Particle bonding mechanisms
During the sintering process, particle bonding occurs, resulting in the cohesion and densification of the material. Several particle bonding mechanisms contribute to this phenomenon.
The key particle bonding mechanisms involved in sintering are as follows:
1. Solid-State Diffusion: Solid-state diffusion is one of the primary bonding mechanisms in sintering. At elevated temperatures, atoms or ions migrate across the surfaces of adjacent particles, leading to atomic rearrangement and the formation of bonds. This diffusion of species allows for the gradual merging of particles and the establishment of interparticle contacts.
2. Surface Diffusion: Surface diffusion involves the movement of atoms or molecules along the surfaces of particles. It plays a significant role in sintering, particularly during the early stages when particles start to come into contact. Surface diffusion facilitates atomic migration, promoting the growth of necks or bridges between particles.
3. Plastic Deformation: Under applied pressure, plastic deformation can occur, particularly in materials with ductile characteristics. The particles can undergo plastic flow and deformation, enabling interlocking and bonding between neighboring particles. This mechanism is common in metal powder sintering processes.
4. Localized Melting: Localized melting or partial melting can occur during sintering. This happens when the temperature reaches a point where the material experiences localized softening or melting at particle contact points. The molten material acts as a bridge, facilitating the bonding between particles. Upon cooling, the material solidifies, forming strong bonds.
It is important to note that different bonding mechanisms may predominate depending on factors such as material composition, temperature, pressure, and particle characteristics. The combination of these bonding mechanisms during the sintering process contributes to the densification and structural development of the material, resulting in improved mechanical strength and other desired properties.
Understanding and controlling these particle bonding mechanisms are crucial for optimizing the sintering process parameters and achieving the desired material properties. The choice of sintering conditions, such as temperature, time, pressure, and atmosphere, can influence the prevalence and effectiveness of these bonding mechanisms, ultimately impacting the quality and characteristics of the sintered product.
Difference between Calcination and Sintering
Calcination and sintering are two distinct thermal treatment processes that are used for different purposes and result in different outcomes.
Here are the key differences between calcination and sintering:
1. Purpose:
• Calcination: The main purpose of calcination is to drive off volatile components, remove impurities, or induce chemical transformations in a material. It is often used to produce oxides, dehydrate materials, or convert unstable compounds into stable forms.
• Sintering: Sintering aims to consolidate and densify powdered or granulated materials, such as metal powders or ceramics, by bonding particles together. The primary goal is to achieve a cohesive solid mass with improved mechanical strength, reduced porosity and enhanced structural integrity.
2. Temperature Range:
• Calcination: Calcination typically occurs at high temperatures, generally in the range of 700 to 1000 degrees Celsius or higher, depending on the material and desired reactions.
• Sintering: Sintering generally occurs at temperatures below the melting point of the material being processed. The specific temperature range varies but can typically range from several hundred to around 1600 degrees Celsius, depending on the materials and their properties.
3. Chemical Reactions:
• Calcination: Calcination involves various chemical reactions, such as dehydration, decarbonization, and decomposition. These reactions lead to the removal of volatile components or the transformation of compounds into different forms.
• Sintering: Sintering primarily relies on the diffusion and bonding of particles without significant chemical reactions. It involves solid-state diffusion, surface diffusion, plastic deformation, and, in some cases, localized melting. The bonding mechanisms result in the densification and cohesion of the material.
4. Particle Behavior:
• Calcination: Calcination focuses on the chemical and physical transformations of the material. It often leads to the removal of volatile components, changes in crystal structures, or the formation of new compounds. The particles may change composition and structure.
• Sintering: Sintering involves the consolidation and bonding of particles to form a cohesive mass. The particles may undergo particle rearrangement, bonding, and grain growth, leading to increased density and improved mechanical properties.
5. Application:
• Calcination: Calcination is used in various industries, including mineral processing, cement production, and chemical manufacturing. It is employed to achieve specific chemical transformations, remove impurities, or produce materials with desired properties.
• Sintering: Sintering finds applications in industries such as metallurgy, ceramics, and powder metallurgy. It is used to produce solid components or materials with improved strength, density, and structural integrity. Sintering is commonly employed for manufacturing metal components, ceramic products, and composite materials.
Understanding the difference between calcination and sintering is crucial for selecting the appropriate thermal treatment process for a specific material and desired outcomes. While both processes involve heat treatment, they have distinct purposes, temperature ranges, chemical reactions, and particle behavior, leading to different material transformations and end products.
Comparison Chart
Here’s a comparison chart highlighting the key differences between calcination and sintering:
| Calcination | Sintering | |
|---|---|---|
| Purpose | Drive off volatile components, remove impurities, induce chemical transformations | Consolidate and densify powdered materials, bond particles together |
| Temperature Range | High temperatures, typically above 700°C | Below is the melting point of the material |
| Chemical Reactions | Involves chemical reactions and transformations | Primarily involves particle bonding mechanisms |
| Particle Behavior | Chemical and physical transformations | Particle rearrangement, bonding, and grain growth |
| Application | Minerals processing, cement production, chemical manufacturing | Metallurgy, ceramics, powder metallurgy, production of metal components and ceramic products |
| Key Outcome | Removal of volatile components, changes in crystal structures, formation of new compounds | Increased density, improved mechanical properties, the cohesion of particles |
| Main Mechanism | Chemical reactions | Particle bonding mechanisms |
It’s important to note that while this chart provides a general overview of the differences between calcination and sintering, there may be variations and exceptions depending on specific materials, processes, and desired outcomes.
Conclusion
The distinction between calcination and sintering lies in their objectives and outcomes. Calcination primarily focuses on the removal of volatile components through heat-induced chemical transformations, while sintering aims to enhance the strength and density of materials through particle bonding.
Both processes play vital roles in materials science and engineering, finding applications in diverse industries. Understanding the dissimilarities between calcination and sintering enables scientists and engineers to make informed decisions regarding the appropriate treatment for specific materials, leading to the development of improved products and technologies.
