Importance of understanding the difference between Molecule of Element and Molecule of Compound
Understanding the difference between a Molecule of Element and Molecule of Compound is crucial for several reasons:
Chemical Composition: By distinguishing molecules of elements and compounds, we can quickly ascertain what kinds of atoms exist within any substance. This knowledge is fundamental to understanding the composition and structure of matter.
Chemical Reactions: Molecules of elements and compounds behave differently in chemical reactions. Elements often form molecules to achieve a stable configuration, while compounds result from the combination of different elements. Understanding the nature of these molecules is essential for predicting and explaining chemical reactions and their outcomes.
Physical Properties: Molecules of elements and compounds possess distinct physical properties. Elements possess specific properties determined by the arrangement and behavior of their atoms, including boiling and melting points, density, and electrical conductivity. Compounds exhibit physical characteristics which differ significantly from their constituent elements. Recognizing these differences aids in predicting and manipulating the behavior of substances.
Biological Systems: Living organisms are composed of complex molecules, including both elements and compounds. Understanding differences at a molecular scale is fundamental for understanding biological processes like metabolism and cell functions.
Material Science and Engineering: The distinction between molecules of elements and compounds is relevant in material science and engineering. Different molecules exhibit varying properties, including strength, conductivity, magnetism, and optical characteristics. This understanding enables the design and development of new materials with specific properties for various applications.
Environmental and Industrial Applications: Recognizing the difference between molecules of elements and compounds is crucial for environmental and industrial applications. Identification and analysis of pollutants allow us to better understand their role within ecosystems as well as devise effective remediation and pollution control strategies. In industrial processes, knowledge of molecule types is essential for optimizing production, ensuring product quality, and minimizing adverse environmental impacts.
Understanding the distinctions between a Molecule of Element and Molecule of Compound provides a basis for comprehending the composition, behavior, and properties of substances. Knowledge has broad-reaching effects across scientific, technological, and practical areas – deepening our understanding of nature while opening doors for advancement in various areas.
Definition of Molecule of Element
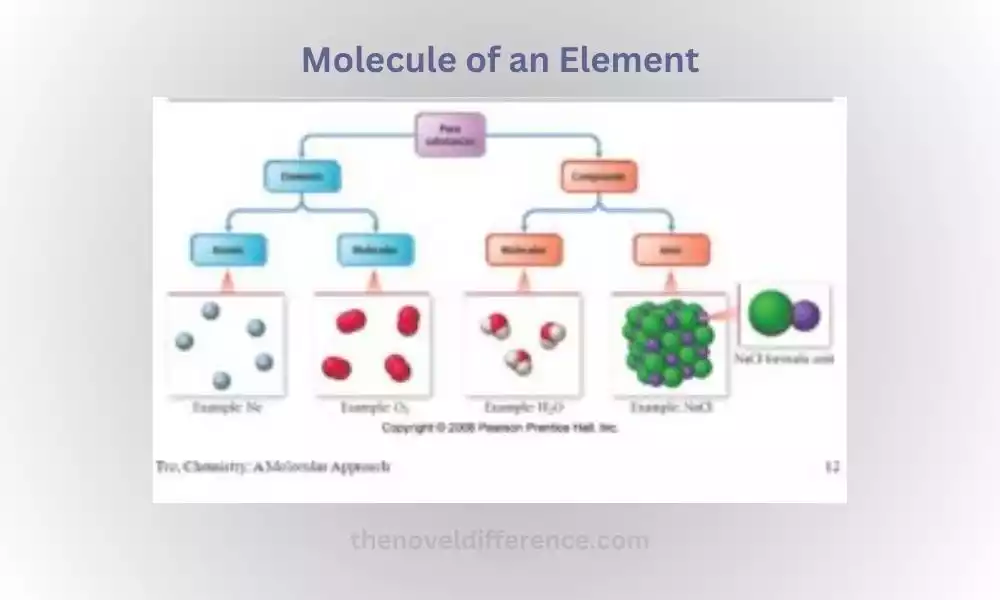
Its Molecules can be defined as groups of two or more atoms chemically bound together by chemical bonds; it represents the smallest unit that maintains all its original chemical properties and maintains its structure over time. Molecules may comprise elements belonging to either family.
Molecules take many shapes. From diatomic molecules like oxygen (O2) and hydrogen (H2) to more intricate molecules like proteins or DNA. Chemical bonds hold together these constituent atoms within molecules; covalent, ionic metallic bonding may all exist depending on which elements make up its makeup.
Molecules’ physical and chemical properties depend upon how their constituent atoms arrange and interact, with each determining its shape, size, chemical behavior, and role within biological, chemical, or physical processes. Understanding molecules’ properties are paramount in fields like chemistry, biology, pharmacology, and materials science.
What is the Molecule of an Element?
A molecule of an element refers to any grouping of two or more atoms from that element that have chemically joined together, creating bonds. While elements are typically represented by single atoms (such as oxygen, O, or hydrogen, H), some elements can exist as molecules due to the nature of their atomic bonding.
Molecules of elements may take various shapes depending on both their elements and the conditions under which they were produced. Some elements exist as diatomic molecules, meaning they consist of two atoms bonded together. Oxygen (O2) and nitrogen (N2) are examples of diatomic elements. These molecules are stable and commonly encountered in nature.
Other elements can form molecules with larger numbers of atoms, such as sulfur (S8) and phosphorus (P4), which exist as octatomic and tetrahedral molecules, respectively. These molecules consist of multiple atoms of the same element bonded together in a specific arrangement.
It is important to note that not all elements form molecules under normal conditions. Some elements, known as noble gases (such as helium, neon, and argon), exist as single atoms and do not readily form stable molecules due to their stable electron configurations.
The formation of molecules of elements is governed by the principles of chemical bonding, primarily through the sharing of electrons. These molecules can exhibit unique properties and behaviors, including specific melting and boiling points, reactivity, and physical characteristics.
Understanding molecules of elements is crucial in various scientific disciplines, including chemistry, physics, and materials science. It provides insights into the properties, behavior, and reactivity of specific elements, enabling the prediction and explanation of chemical reactions and the design of new materials with tailored characteristics.
Definition of Molecule of Compound
A compound is defined as any substance composed of multiple different elements chemically combined together in fixed proportions, bound together through chemical bonds to produce its unique set of properties and qualities.
Compounds contain a unique chemical formula that specifies the ratio of elements present within them and thus provides insight into what kinds and quantities of elements make up each compound, such as water (H2O). For instance, water comprises two hydrogen atoms and one oxygen atom, providing information regarding the types and amounts present.
Compounds can be created through many chemical reactions, including synthesis, decomposition, or rearrangement of atoms. Their bonds between their constituent atoms may be covalent, ionic, or metallic in nature or combinations thereof.
Different from mixtures, in which physical substances combine and retain their individual properties, compounds have distinctive features distinct from their constituent elements. When elements come together to form compounds, their properties often undergo dramatic shifts such as melting point, boiling point, reactivity, or appearance changes as part of this new entity.
Compounds play an essential part in chemistry and everyday life. From pharmaceuticals and plastics to fertilizers and dyes – compounds form the backbone for numerous products in various scientific, industrial, and everyday applications.
Being aware of their properties is vital in designing processes more effectively while developing novel materials to advance scientific knowledge in disciplines like chemistry, biology, and materials science.
What is the Molecule of a Compound?
A molecule of a compound refers to any grouping of two or more atoms from different elements that chemically bond together into an inert substance with distinct properties and unique qualities, unlike elements, which consist of all identical atoms bonded in specific ratios to form one substance with unique qualities.
Compound molecules are held together through chemical bonds of various kinds – covalent, ionic, or metallic bonds can all bind the atoms in different combinations that determine their structure and properties. The bond type between each pair of atoms determines the shape and properties of the compound.
Molecules of compounds come in all shapes and sizes. Some molecules, like H2O, CO2, or CH4, consist of only three or four atoms that bond together while other substances like proteins, carbohydrates, or DNA possess intricate structures with numerous bonds between atoms bonded tightly together.
Compound properties vary significantly from those of its constituent elements. When combined, those elements’ individual properties may change and new characteristics emerge – for instance when sodium (Na) and chlorine (Cl) chemically react and form sodium chloride (NaCl), an entirely unique compound emerges with unique features – like its characteristic taste in table salt!
Understanding molecules of compounds is integral for various scientific fields, including chemistry, biology, pharmacology, and materials science. Understanding molecules allows scientists to study their structures, behavior, and reactivities as well as predict chemical reactions for specific applications or create new molecules with desirable characteristics for new purposes.
Molecules of compounds also play an essential role in everyday life. We encounter medications, plastics, detergents, and fertilizers that contain compounds composed of multiple elements bonded together – understanding these molecules can assist designers and improve functionality, safety, and environmental impacts for products like these.

Understanding molecules of compounds is critical for studying their properties, studying chemical reactions, and creating innovative materials and technologies.
Differences between Molecules of an Element and Molecules of a compound
Understanding the fundamental differences between an elemental molecule and a compound molecule is dependent on knowing their difference.
Here are some key differences between the Molecule of Element and Molecule of Compound:
Composition:
Molecules: Composed of two or three atoms that have been bound, molecules have many purposes in daily life and must function optimally to perform these duties effectively. Two oxygen atoms make up molecular oxygen, for example.
Molecules of Compounds: They are composed of two or more atoms from different elements. Water (H2O) contains two hydrogen and one oxygen atom.
Chemical bonding:
Molecules of an Element: The bonds in molecules of elements may be covalent, metal, or other types of bonding specific to the element. Examples of covalent bonds include oxygen (O2) and metallic bonds in sodium elemental (Na).
Compound Molecule: Compound molecules involve different types of chemical bonds, such as covalent, metallic, or ionic bonding depending on the compound. Example of covalent bond formation between sodium and chlorine elements in NaCl: it forms between its elements when formed as NaCl.
Physical properties:
Molecules of an Element: The physical properties of elements are determined by their atomic structure. At room temperature and pressure, the oxygen (O2) diatomic molecule is a colorless liquid.
Molecules of a compound: Many compounds exhibit physical properties different from those of their constituent elements. Water (H2O), for instance, remains liquid under normal pressure and temperature conditions even though its components include hydrogen and oxygen atoms.
Examples & Characteristics:
Diatomic elements: Diatomic elements such as hydrogen (H2) or oxygen (O2) constitute examples of molecules. The molecules of an element can occur naturally or be formed under certain conditions.
Molecules of Compounds: Examples are water (H2O), CO2 (CO2), and methane(CH4). Compounds form through chemical reactions.
Understanding these differences can be useful for many scientific and practical purposes, including predicting chemical reactions and studying substances’ properties, designing materials, and analyzing biological systems.
Scientists can learn more about the characteristics, behavior, and reactivity of substances by recognizing the difference between elements and compounds.
Molecule of Element and Molecule of Compound in Tabular Form
Here’s a tabular comparison highlighting the differences between a molecule of an element and a molecule of a compound:
| Aspect | Molecule of an Element | Molecule of a Compound |
|---|---|---|
| Composition | Two or more atoms of the same element | Two or more atoms of different elements |
| Chemical Bonding | Covalent, metallic, or element-specific | Ionic, covalent, metallic, or compound-specific |
| Physical Properties | Unique physical properties of the element | Physical properties may differ from constituent elements |
| Examples | Oxygen (O2), hydrogen (H2), nitrogen (N2) | Water (H2O), carbon dioxide (CO2), methane (CH4) |
The table summarizes the contrasting features of molecules of elements and compounds, highlighting their composition, types of chemical bonding, physical properties, and examples.
What are the similarities between Molecule of Element and Molecule of Compound?
While molecules of elements and compounds have distinct differences, there are some similarities between them as well. Here are a few similarities:
- Atomic Structure: Both molecules of elements and compounds consist of atoms. Atoms are the basic building blocks of matter, and they are present in both types of molecules.
- Chemical Bonding: Atoms in molecules are joined through chemical bonds to one another through chemical bonding reactions, keeping their structure together and functioning as intended. While the types of bonding may differ, such as covalent or metallic bonding in elements and ionic or covalent bonding in compounds, the concept of atoms forming bonds to create stable structures is a common characteristic.
- Formation: Molecules of both elements and compounds can be formed through chemical reactions. Elements can combine with other elements or with themselves to form molecules, while compounds are formed when atoms of different elements chemically react and bond together.
- Structural Representation: Molecules of both elements and compounds can be represented using chemical formulas or structural formulas. Chemical formulas provide information about the types and ratios of atoms present in the molecule, allowing for a concise representation of its composition.
- Interaction and Reactivity: Both types of molecules can interact and participate in chemical reactions. Molecules of elements can undergo reactions to form different compounds or participate in redox reactions, while molecules of compounds can undergo various types of chemical transformations or reactions.
It’s important to note that while there are similarities, the distinct differences between molecules of elements and compounds ultimately define their unique characteristics, behavior, and properties.
Conclusion
Understanding the distinctions between an element molecule and a compound molecule is integral to understanding the composition, behavior, and properties of substances.
A molecule of an element consists of two or more atoms of the same element chemically bonded together. These molecules can exist as diatomic molecules or have more complex structures, depending on the element involved. Molecules of elements exhibit specific physical properties and behavior determined by their atomic bonding.
Conversely, molecules of compounds consist of multiple elements chemically linked together into one molecule. Compounds have distinct chemical formulas representing the ratio of atoms present.
Molecules of compounds possess unique properties different from their constituent elements. Compounds play a fundamental role in various scientific, industrial, and everyday applications.
By understanding the differences between molecules of elements and compounds, scientists and researchers can predict and explain chemical reactions, study the properties of substances, design new materials, and analyze biological systems.
