Organic chemistry distinguishes Iso and Sec in Organic Chemistry based on whether all carbon atoms but one form a continuous chain; on the other hand, Sec compounds include functional groups bonded directly or indirectly to secondary carbon atoms.
Definition of Iso and Sec in Organic Chemistry
ISO in organic chemistry: Organic chemists frequently refer to “ISO” as an abbreviation for “isopropyl” or “isopropylidene.” It refers to an arrangement in a molecule where one carbon atom connects directly with two others forming an extended network-branched structure.
ISO refers to an abbreviation for equal and identical, reflecting that two carbon atoms connected by bondings are equivalent and thus represent equivalent units of carbon content.
ISO compounds are characterized by the presence of the isopropyl group (-CH(CH3)2) or its variations. This group consists of a central carbon atom bonded to two methyl groups (CH3) and one hydrogen atom.
The isopropyl group can be attached to various molecular frameworks, giving rise to different ISO compounds with distinct chemical and physical properties.
The ISO prefix is commonly used in chemical nomenclature to designate the presence of the isopropyl group in a molecule. It helps in identifying and differentiating ISO compounds from other structural isomers.
The presence of the ISO group can influence the reactivity, solubility, and biological activity of the compound, making it an important consideration in organic chemistry research and applications.
SEC in organic chemistry: “SEC” in organic chemistry refers to “secondary,” or sec-butyl. This refers to an arrangement in a molecule in which one carbon atom connects directly with two other carbon atoms forming an “S” shape structure, creating a branching formation.
SEC is an abbreviation for “second” or “following”, denoting that an attached carbon atom represents the second carbon in an otherwise branching structure.
SEC compounds are characterized by the presence of the sec-butyl group (-CH2CH(CH3)2) or its variations. This group consists of a central carbon atom bonded to two methyl groups (CH3) and one ethyl group (CH2CH3).
The sec-butyl group can be attached to various molecular frameworks, giving rise to different SEC compounds with distinct chemical and physical properties.
The SEC prefix is commonly used in chemical nomenclature to designate the presence of the sec-butyl group in a molecule. It helps in identifying and differentiating SEC compounds from other structural isomers.
The presence of the SEC group can influence the reactivity, solubility, and biological activity of the compound, making it an important consideration in organic chemistry research and applications.
Importance of understanding the difference between ISO and SEC
Understanding the difference between Iso and Sec in Organic Chemistry is important in organic chemistry for several reasons:
Structural Identification: Iso and Sec in Organic Chemistry have different structural arrangements of atoms, particularly in the branching pattern. Distinguishing between these two arrangements is crucial for accurately identifying and classifying organic molecules. The ability to differentiate between Iso and Sec in Organic Chemistry allows chemists to describe and communicate the structure of organic compounds effectively.
Nomenclature: The ISO and SEC prefixes are used in chemical nomenclature to indicate the presence of specific structural groups in a molecule. Knowing the difference between ISO and SEC is essential for correctly naming and classifying compounds. Accurate nomenclature ensures clear communication and facilitates the understanding and interpretation of chemical structures.
Reactivity and Properties: The structural difference between Iso and Sec in Organic Chemistry can significantly influence their reactivity and physical properties. Iso and Sec in Organic Chemistry may exhibit distinct chemical behaviors, including different reaction rates, steric hindrance effects, and interactions with other molecules. Understanding these differences allows chemists to predict and manipulate the behavior and properties of Iso and Sec in Organic Chemistry, enabling rational design and synthesis of organic molecules.
Pharmaceutical and Biological Applications: Iso and Sec in Organic Chemistry are prevalent in natural products and pharmaceuticals. Their distinct structural features can affect biological activity, pharmacokinetics, and drug interactions. By understanding the difference between Iso and Sec in Organic Chemistry, researchers can study and optimize the biological effects of these compounds, leading to the development of more effective drugs and therapeutic interventions.
Synthetic Strategies: The knowledge of Iso and Sec in Organic Chemistry aids in designing synthetic routes and strategies for the construction of specific molecular frameworks. Differentiating between ISO and SEC building blocks is crucial for choosing appropriate starting materials and controlling the stereochemistry and regiochemistry of the synthesized compounds. Understanding the difference between Iso and Sec in Organic Chemistry enables chemists to plan and execute efficient syntheses with desired structural features.
Understanding the difference between Iso and Sec in Organic Chemistry is vital for structural identification, accurate nomenclature, prediction of reactivity and properties, exploration of pharmaceutical and biological applications, and efficient synthetic strategies in organic chemistry.
It enhances our ability to comprehend, manipulate, and utilize organic compounds in various scientific and practical contexts.
ISO Compounds
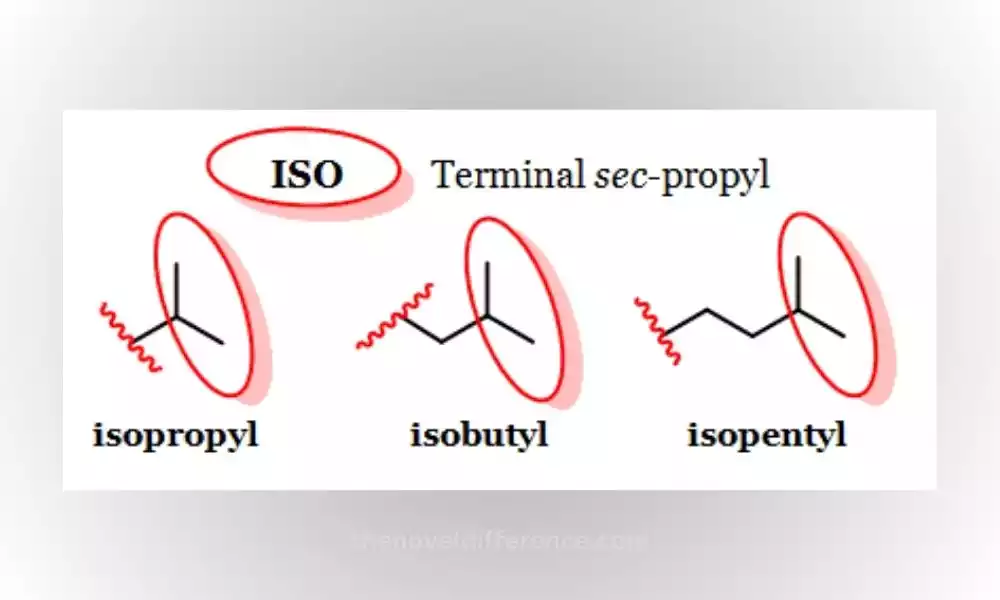
ISO compounds are organic compounds that contain the isopropyl group (-CH(CH3)2) or their variations. Isopropyl groups consist of one central carbon atom linked by three methyl groups (CH3). There is one hydrogen atom as well.
This group is attached to different molecular frameworks, resulting in a variety of ISO compounds with distinct chemical and physical properties.
ISO compounds can be distinguished from one another by their branching structure, where one carbon atom connects with two other carbon atoms at its center.
This branching arrangement differentiates ISO compounds from straight-chain or linear compounds. Isopropyl groups can have dramatic consequences on the reactivity, solubility, and biological activity of compounds.
ISO compounds can be found in many natural products, pharmaceutical drugs, and industrial chemicals. Some examples of ISO compounds include:
Isopropyl alcohol (2-propanol): A common solvent and disinfectant.
Isopropylamine: Used in the synthesis of pharmaceuticals, dyes, and pesticides.
Isopropyl acetate: A solvent and flavoring agent in the food industry.
Isopropyl bromide: Used as an alkylating agent in organic synthesis.
Isopropylbenzene (cumene): A precursor in the production of phenol and acetone.
ISO compounds often exhibit unique properties due to their branching structure, such as altered boiling points, melting points, and reactivity compared to their linear counterparts.
Understanding ISO compounds and their distinct characteristics is essential in organic chemistry for identification, synthesis, and understanding of their roles in various applications.
SEC Compounds
SEC compounds, in the context of organic chemistry, refer to organic compounds that contain the sec-butyl group (-CH2CH(CH3)2) or its variations. “SEC” is an abbreviation for “secundus,” Latin for “second.” This designation indicates that the carbon atom attached is second in the branching structure.
The sec-butyl group consists of a central carbon atom bonded to two methyl groups (CH3) and one ethyl group (CH2CH3). This group can be attached to different molecular frameworks, resulting in a variety of SEC compounds with distinct chemical and physical properties.
SEC compounds are characterized by their branched structure, where the central carbon atom is attached to two other carbon atoms. This branching arrangement differentiates SEC compounds from straight-chain or linear compounds.
The presence of the sec-butyl group can have significant effects on the reactivity, solubility, and biological activity of the compound.
SEC compounds are encountered in various fields, including organic synthesis, pharmaceuticals, flavors and fragrances, and industrial applications. They can serve as building blocks or functional groups in the design and synthesis of complex organic molecules.
In chemical nomenclature, the SEC prefix is used to designate the presence of the sec-butyl group in a compound. This prefix helps in identifying and differentiating SEC compounds from other structural isomers, allowing for clear communication and accurate representation of their molecular structures.
Understanding SEC compounds and their unique characteristics is important in organic chemistry for identification, synthesis, and exploring their roles in various applications.
The presence of the sec-butyl group can significantly influence the reactivity, properties, and biological activity of these compounds, making them of interest to researchers and chemists.
What is the Difference Between Iso and Sec in Organic Chemistry
The difference between Iso and Sec in Organic Chemistry lies in the specific structural arrangements of the carbon atoms to which the respective functional groups are attached.
Here’s a breakdown of the key differences:
ISO Compounds:
Structure: ISO compounds have a branched structure in which a central carbon atom is attached to two other carbon atoms. The isopropyl group (-CH(CH3)2) is attached to this central carbon atom.
Examples: Isopropyl alcohol, isopropylamine, and isopropyl acetate.
SEC Compounds:
Structure: SEC compounds also have a branched structure, but in this case, a central carbon atom is attached to two other carbon atoms. The sec-butyl group (-CH2CH(CH3)2) is attached to this central carbon atom.
Examples: Sec-butyl alcohol, sec-butylamine, sec-butyl acetate.
The main difference between Iso and Sec in Organic Chemistry is the specific functional group attached to the central carbon atom. ISO compounds have the isopropyl group (-CH(CH3)2), while SEC compounds have the sec-butyl group (-CH2CH(CH3)2).
These differences in functional groups can lead to variations in the chemical and physical properties of Iso and Sec in Organic Chemistry, including differences in reactivity, boiling points, solubility, and biological activity.
It’s important to note that the ISO and SEC prefixes are used in chemical nomenclature to indicate the presence of these specific functional groups in a compound. Understanding these differences allows for accurate identification, naming, and classification of organic compounds.
Iso vs Sec in Organic Chemistry in Tabular Form
Sure! Here’s a tabular comparison highlighting the key differences between Iso and Sec in Organic Chemistry in organic chemistry:
| ISO Compounds | SEC Compounds | |
|---|---|---|
| Structure | Branched | Branched |
| Functional Group | Isopropyl group (-CH(CH3)2) | Sec-butyl group (-CH2CH(CH3)2) |
| Carbon Atom Connectivity | Central carbon is attached to two other carbon atoms | Central carbon is attached to two other carbon atoms |
| Naming Prefix | ISO | SEC |
| Examples | Isopropyl alcohol, isopropylamine, isopropyl acetate | Sec-butyl alcohol, sec-butylamine, sec-butyl acetate |
| Key Characteristics | – Isopropyl group attached to central carbon atom – Two methyl groups and one hydrogen atom in the isopropyl group |
– Sec-butyl group attached to central carbon atom – Two methyl groups and one ethyl group in the sec-butyl group |
| Typical Properties | – May exhibit altered reactivity, boiling points, and solubility compared to linear compounds – Isopropyl group affects the biological activity of compounds |
– May exhibit altered reactivity, boiling points, and solubility compared to linear compounds – Sec-butyl group affects the biological activity of compounds |
Please note that this table provides a general overview and some common examples of Iso and Sec in Organic Chemistry. The properties and characteristics can vary depending on the specific compounds involved.
what are the similarities between Iso and Sec in Organic Chemistry?
Although Iso and Sec in Organic Chemistry have distinct structural differences, there are a few similarities between them in organic chemistry.
Here are some similarities:
Branched Structure: Both Iso and Sec in Organic Chemistry have a branched structure, where a central carbon atom is attached to two other carbon atoms. This branching arrangement distinguishes them from straight-chain or linear compounds.
Alkyl Groups: Both Iso and Sec in Organic Chemistry contain alkyl groups. ISO compounds have the isopropyl group (-CH(CH3)2), while SEC compounds have the sec-butyl group (-CH2CH(CH3)2). Alkyl groups are important functional groups in organic chemistry and contribute to the properties and reactivity of the compounds.
Chemical Reactivity: Iso and Sec in Organic Chemistry can exhibit similar chemical reactivity due to the presence of alkyl groups. For example, they may undergo similar substitution or elimination reactions. The reactivity can be influenced by factors such as neighboring functional groups and the electronic properties of the alkyl groups.
Organic Synthesis: Both Iso and Sec in Organic Chemistry can serve as building blocks or starting materials in organic synthesis. They can be used to introduce specific functional groups or branches into target molecules during the synthesis process.
Industrial Applications: Iso and Sec in Organic Chemistry find applications in various industrial sectors, including pharmaceuticals, flavors and fragrances, and chemical manufacturing. Their unique structural features and properties make them valuable in these industries.
It’s important to note that while Iso and Sec in Organic Chemistry share these similarities, they also have distinct differences in terms of the specific functional groups and carbon atom connectivity. These differences lead to variances in chemical and physical properties, nomenclature, and biological activities of plants.
Biological Significance and Applications
Both Iso and Sec in Organic Chemistry can have biological significance and find applications in various fields. Here are some examples:
Pharmaceutical Industry: Iso and Sec in Organic Chemistry are often used as building blocks in the synthesis of pharmaceutical drugs. Drugs possess unique structural elements which may influence the bioavailability, pharmacokinetics, and biological activity of their target drug(s). Iso and Sec in Organic Chemistry can serve as starting materials for the synthesis of active pharmaceutical ingredients (APIs) or be incorporated into the drug molecule to enhance its properties.
Natural Products: Many natural products contain Iso and Sec in Organic Chemistry. These compounds can contribute to the biological activity and medicinal properties of plants and microorganisms. Researchers study and isolate natural products to explore potential medicinal applications, including antimicrobial, antifungal, or even anticancer agents.
Flavors and Fragrances: Iso and Sec in Organic Chemistry are commonly used in the flavor and fragrance industry. Aromatics add dimension and flavor to many food, beverage, and perfume products. Iso and Sec in Organic Chemistry with specific odor profiles are utilized to create desired sensory experiences in consumer products.
Chemical Biology: Iso and Sec in Organic Chemistry can be employed in chemical biology research to study biological processes and pathways. By designing and synthesizing compounds with ISO or SEC groups, researchers can investigate their interactions with biological targets and understand their effects on cellular systems. These studies can provide insights into drug discovery, chemical genetics, and understanding of biological mechanisms.
Pesticides and Agrochemicals: Iso and Sec in Organic Chemistry find applications in the development of pesticides and agrochemicals. Their structural features can influence the efficacy, selectivity, and environmental impact of these compounds. Iso and Sec in Organic Chemistry may act as active ingredients or functional components in pesticide formulations for pest control and crop protection.
Material Science: Iso and Sec in Organic Chemistry can be utilized in material science applications. They can also be combined with polymers or resins to modify properties like adhesion, durability, and thermal stability of polymeric structures or coatings. Iso and Sec in Organic Chemistry can enhance the performance and functionality of materials in various industrial sectors.
Iso and Sec in Organic Chemistry have biological significance and diverse applications in pharmaceuticals, natural products, flavors and fragrances, chemical biology, agrochemicals, and material science. Their unique structural features and properties make them valuable in these areas of research and industry.
Conclusion
Understanding the difference between Iso and Sec in Organic Chemistry is essential in organic chemistry. ISO compounds contain the isopropyl group (-CH(CH3)2), while SEC compounds contain the sec-butyl group (-CH2CH(CH3)2). These compounds have distinct structural arrangements and functional groups, leading to variations in their chemical and physical properties.
Knowing the difference between Iso and Sec in Organic Chemistry is crucial for accurate structural identification, nomenclature, and classification of organic molecules. It enables chemists to predict and manipulate their reactivity, properties, and biological activities. Iso and Sec in Organic Chemistry are important in pharmaceuticals, natural products, flavors and fragrances, chemical biology, agrochemicals, and material science.
