When discussing molecular structures, physical courses of action, or indeed the format of program frameworks, terms like compliance and setup are commonly experienced. These terms play vital parts in chemistry, science, computer science, and numerous other disciplines.
Understanding the subtleties between these two concepts is basic to picking up bits of knowledge about their applications and centrality. We’ll dig into the distinction between compliance and setup, investigating each term in detail, and shedding light on their particular highlights and applications.
Definition of Compliance and Arrangement
Conformation: Conformation refers to the spatial course of action of iotas in a particle, especially concerning the turn of single bonds. It bargains with the distinctive three-dimensional shapes a molecule can receive while keeping up the same network of its iotas. It describes the different ways a molecule can twist and bend without breaking any bonds. Conformations are crucial in understanding atomic behavior, solidness, and reactivity, particularly in areas like chemistry, organic chemistry, and atomic science.
Configuration: Configuration Refers to the spatial arrangement of atoms in a molecule that cannot be interconverted by rotation around single bonds. It is related to the stereochemistry of a particle and includes the assurance of the settled positions of its substituents in three-dimensional space. Configuration is concerned with the course of action of iotas that comes about in stereoisomers, which are atoms with the same chemical equation and network but vary in their spatial course of action. Configurational isomers cannot be converted into each other without breaking covalent bonds and are distinguished by their optical activity or biological properties.
Conformation deals with flexible molecular shapes due to rotation around single bonds, while configuration deals with the fixed spatial arrangement of atoms that gives rise to stereoisomers. Both conformation and configuration play essential roles in understanding molecular properties, interactions, and behaviors across various scientific fields.
Importance of understanding these concepts in various fields
Understanding the concepts of conformation and configuration is of fundamental significance in different logical areas due to the following reasons:
1. Chemistry: Understanding conformational changes is vital for foreseeing and clarifying atomic reactivity, solidness, and response instruments. It makes a difference when chemists plan and optimize drugs, think about response energy, and investigate the behavior of complex particles.
2. Biochemistry: Compliance plays a key part in their capacities. Information on protein conformation is fundamental in medicating plans, understanding protein catalysis, and anticipating protein-protein intelligence. Essentially, understanding the configuration of chiral atoms is crucial in examining organic forms, as enantiomers can have endlessly distinctive organic impacts.
3. Molecular Biology: Conformational changes in DNA and RNA are noteworthy in quality expression, control, and replication. Understanding these changes is vital for disentangling atomic forms and creating gene-targeted treatments.
4. Material Science: Conformational arrangements affect their mechanical, thermal, and optical properties. Information on these conformations is basic in planning materials for particular applications.
5. Drug Design and Pharmaceuticals: Configurational isomers, such as enantiomers, can exhibit different pharmacological activities. Understanding the arrangement of medicate particles is imperative for guaranteeing adequacy, diminishing side impacts, and getting administrative endorsement.
6. Environmental Science: Conformational changes in atoms can impact their interaction with natural variables, such as discussing toxins, driving to varieties in poisonous quality, and natural affect.
7. Computational Modeling: Accurate representation of molecular conformation and configuration is crucial in computational chemistry and molecular simulations. It allows researchers to perform accurate predictions and simulations of molecular behavior and interactions.
8. Food Science: Understanding the configuration of flavor and aroma compounds in food products is essential for quality control, taste enhancement, and developing new food products.
9. Agriculture: Knowledge of the configuration of chiral molecules is essential in understanding pesticide behavior, metabolism, and environmental impact.
10. Nanotechnology: Conformational changes in nanoscale materials can altogether affect their electronic, mechanical, and optical properties, in this way affecting their applications in different businesses.
A comprehensive understanding of conformation and configuration is imperative in various logical areas, empowering analysts and experts to control and utilize atoms viably for different applications, from sedate improvement to materials science and the past.
What is Conformation?
Conformation refers to the spatial arrangement of atoms in a molecule, particularly concerning the rotation around single bonds. It depicts the distinctive three-dimensional shapes an atom can receive while keeping up the same network of its iotas. In less complex terms, adaptation bargains with the diverse ways a particle can bend and twist without breaking any covalent bonds.
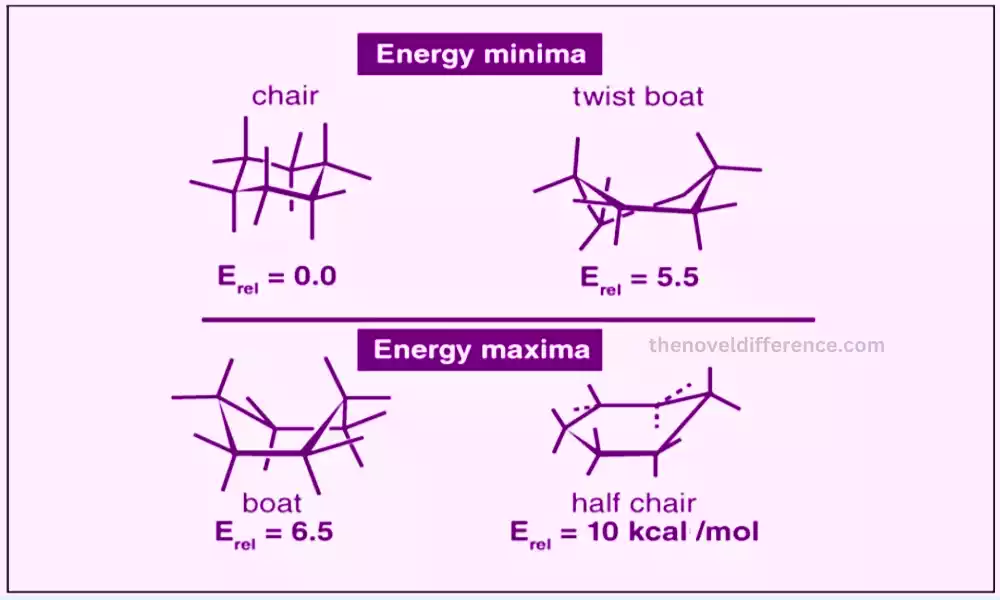
Particles are not inflexible structures; they have adaptability due to the nearness of single bonds that can turn openly. This rotation allows the molecule to adopt different conformations or shapes. It is critical to note that the general chemical equation and network of the molecules stay the same different conformations; as it were the spatial course of action changes.
Conformations are basic in understanding molecular behavior, steadiness, and reactivity. They have critical suggestions in areas such as chemistry, natural chemistry, atomic science, and medication plans. In proteins, different conformations can lead to changes within the protein’s movement or its capacity to connect with other atoms. In natural chemistry, the think-about of conformational isomers helps to understand the reactivity and properties of atoms. The conformational investigation may be a basic angle of present-day computational chemistry and atomic recreations because it permits analysts to show and anticipate atomic behavior precisely.
Rotational freedom
Rotational opportunity, also known as a torsional opportunity, alludes to the capacity of molecules or bunches inside a particle to turn around single covalent bonds without breaking the bond itself. In a particle, not all bonds have the same level of rotational flexibility. A few bonds are more adaptable, permitting without charge turn, whereas others are confined, anticipating free revolution due to steric prevention or other components.
Understanding the concept of rotational flexibility is vital in the consideration of atomic conformations. As said previously, conformations depict the diverse three-dimensional shapes an atom can receive while keeping up the same network of its molecules. The ability of atoms or groups to rotate freely around single bonds leads to various conformations that a molecule can assume.
Rotational opportunity plays a noteworthy part in different viewpoints of atomic behavior:
1. Conformational Isomers: Rotational flexibility gives rise to distinctive conformational isomers, which are unmistakable spatial courses of action of molecules within the particle. Conformational isomers are interconvertible without breaking any covalent bonds, and they contribute to the general vitality scene of the atom.
2. Energy Minimization: Molecules tend to adopt conformations that minimize their energy. By rotating around single bonds, a molecule can explore different conformations and eventually stabilize in the lowest energy state.
3. Chemical Reactivity: Different conformations can lead to differences in the reactivity of molecules. Responsive destinations may be uncovered or covered up in numerous conformations, affecting how the particle interatomic with other atoms amid chemical responses.
4. Protein Structure and Function: Proteins are subject to conformational changes, which are basic for their appropriate work. Proteins can switch between distinctive conformations to perform their organic parts, such as chemical catalysis or ligand official.
5. Molecular Simulations: Understanding rotational freedom is essential for accurate modeling and predicting molecular behavior.
The concept of rotational flexibility is essential in understanding the energetic nature of particles and their capacity to investigate diverse shapes and conformations, which is significant in different logical areas, counting chemistry, organic chemistry, and atomic science.
What is Configuration?
Configuration refers to the spatial course of action of molecules in a particle, especially concerning the settled positions of its substituents in three-dimensional space. Not at all like adaptation, the configuration is concerned with the course of action of iotas that cannot be interconverted by turning around single bonds without breaking covalent bonds.
Configuration is a property that remains consistent for a given atom, and it decides the stereoisomers of the particle. Stereoisomers are atoms with the same chemical equation and network of molecules but vary in their spatial course of action. They can exist as distinctive configurational isomers, each having unmistakable physical and chemical properties.
There are two primary sorts of configurational isomers:
1. Geometric Isomers: These are configurational isomers in which iotas or bunches are settled in several positions around a twofold bond or an inflexible ring structure. The restricted rotation around the double bond or the ring results in two distinct geometric isomers. Common examples include cis-trans isomers in alkenes and geometric isomers in cyclic compounds.
2. Optical Isomers (Enantiomers): These are configurational isomers that are mirror images of each other and cannot be superimposed. Enantiomers have the same physical and chemical properties, but for their interaction with polarized light; one enantiomer pivots plane-polarized light clockwise (dextrorotatory or +), whereas the other pivots it counterclockwise (levorotatory or -). Enantiomers are chiral atoms, meaning they need an inside plane of symmetry.
The setup of a particle, particularly within the case of chiral atoms, has critical suggestions in different areas:
• Pharmaceutical and Drug Design: The configuration of chiral medicate particles can altogether affect their organic movement and intuition with natural receptors. Enantiomers of drugs may show diverse pharmacological impacts, and understanding their configuration is vital in sedate improvement and optimization.
• Biochemistry: Chirality is a fundamental aspect of biological molecules like amino acids and sugars. The configuration of chiral centers in these particles influences their intelligence with proteins and other biomolecules.
• Material Science: The configuration of chiral particles in materials can impact their physical properties, such as optical movement, and is significant in planning and understanding the behavior of chiral materials.
Configuration refers to the settled spatial course of action of iotas in an atom that gives rise to stereoisomers, such as geometric isomers and enantiomers. Understanding configuration is fundamental in different logical disciplines, from medicate planning to fabric science and natural chemistry.
Representation in Chemistry
The representation of both conformation and configuration is fundamental for understanding and communicating the spatial course of action of iotas in particles.
Various notations and methods are used to depict these concepts:
Representation of Conformation:
1. Newman Projections: Newman projections are commonly used to illustrate the conformational arrangement of atoms in acyclic molecules. They give a top-down view along a particular bond, showing the relative positions of substituents around that bond. The eclipsed and staggered conformations can be visualized using Newman projections.
2. Sawhorse Projections: Sawhorse projections are an extension of Newman projections and provide a slightly oblique view, showing the relative orientations of substituents around a particular bond.
3. Fischer Projections: Fischer projections are used to represent the conformation of cyclic molecules, particularly for carbohydrates and other biomolecules. They give a two-dimensional representation with vertical and flat lines to delineate the spatial course of action of substituents.
Representation of Configuration:
1. Fischer Projections: As mentioned earlier, Fischer projections are often used to represent the absolute configuration of chiral molecules, especially carbohydrates and amino acids. The vertical and horizontal lines in the Fischer projection indicate the spatial arrangement of substituents around the chiral center.
2. Stereochemical Notations: For geometric isomers, such as cis-trans isomers in alkenes and geometric isomers in cyclic compounds, the geometric configuration is usually denoted using cis/trans or Z/E notations.
3. R and S Notations: The R and S notations, based on the Cahn-Ingold-Prelog priority rules, are used to specify the absolute configuration of chiral centers in molecules. The documentation allows a need for each substituent around the chiral center and decides whether the atom is in an R (rectus) or S (evil) setup.
Stereochemistry and setup are regularly examined utilizing expressive terms such as “enantiomer,” “diastereomer,” “chiral,” “achiral,” “mesa compound,” etc.
These representations and documentation are vital for passing on the spatial courses of action of particles in atoms precisely, supporting an understanding of the properties, reactivity, and natural exercises of distinctive chemical compounds. They too play an imperative part in chemical communication, investigation, and instruction in different branches of chemistry.
Difference between Conformation and Configuration
Conformation and configuration are related concepts in chemistry, but they have distinct meanings and implications:
1. Definition:
• Conformation: Conformation refers to the spatial arrangement of atoms in a molecule, particularly concerning the rotation around single bonds. It portrays the diverse three-dimensional shapes an atom can embrace while keeping up the same network of its particles. Conformations are dynamic and can change due to free rotation around single bonds without breaking covalent bonds.
• Configuration: Configuration, on the other hand, refers to the fixed spatial arrangement of atoms in a molecule that cannot be interconverted by rotation around single bonds without breaking covalent bonds. It is concerned with the stereoisomers of an atom, which have the same chemical equation and network but vary in their spatial course of action.
2. Type of Changes:
• Conformation: Conformational changes are dynamic and reversible; they involve different arrangements of atoms without altering the actual identity of the atoms or breaking any covalent bonds.
• Configuration: Configuration refers to a settled course of action of molecules; it speaks to diverse stereoisomers that cannot be interconverted without breaking covalent bonds.
3. Examples:
• Conformation: Examples of conformational changes include the rotation of methyl groups in alkanes, leading to staggered and eclipsed conformations, or the rotation of single bonds in flexible molecules, resulting in various shapes.
• Configuration: Examples of configuration include geometric isomers (cis-trans isomers) in alkenes, where the relative positions of substituents around a double bond are fixed, and enantiomers, which are mirror-image stereoisomers that cannot be superimposed.
4. Bond Breaking:
• Conformation: Conformational changes involve only rotations around single bonds without breaking covalent bonds.
• Configuration: Configurational changes require the breaking and remaking of covalent bonds to interconvert between different stereoisomers.
5. Representations:
• Conformation: Conformational changes are often depicted using Newman projections, sawhorse projections, or other methods that show the spatial arrangements of atoms around single bonds.
• Configuration: Configuration is represented using Fischer projections, R and S notations, or cis-trans (Z/E) notations for geometric isomers.
6. Impact on Properties:
• Conformation: Different conformations may have slight variations in molecular properties, such as stability, reactivity, and intermolecular interactions.
• Configuration: Different configurations, especially in chiral molecules, can result in distinct physical properties, biological activities, and interactions with other molecules.
Conformation relates to the adaptable, energetic courses of action of molecules in a particle, whereas configuration depicts the settled spatial courses of action that allow rise to diverse stereoisomers. Both concepts play pivotal parts in understanding atomic behavior, properties, and intuition in different logical disciplines.
Experimental Techniques and Computational Methods
Experimental Techniques and Computational Strategies are utilized in chemistry and related areas to think about and analyze particles, their structures, properties, and behaviors. Both approaches have their qualities and complement each other to supply a comprehensive understanding of chemical frameworks.
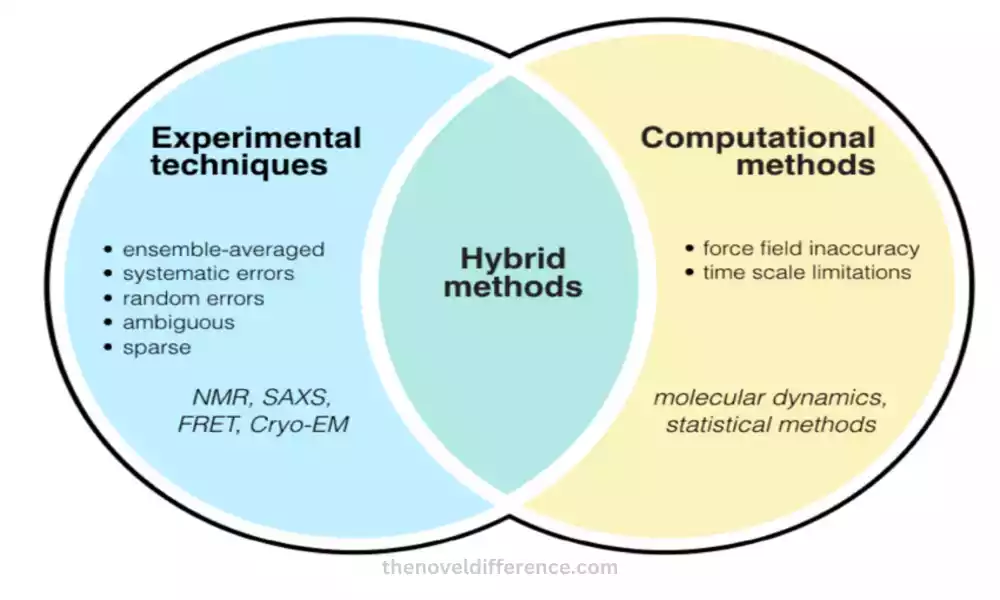
Here are a few common exploratory procedures and computational strategies:
Exploratory Methods:
1. X-ray Crystallography: X-ray crystallography could be a capable exploratory strategy utilized to decide the three-dimensional nuclear structure of crystalline materials, counting little particles and huge biomolecules like proteins and nucleic acids.
2. Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy gives nitty gritty information almost the atomic structure, flow, and intuition of atoms in the arrangement. It is particularly profitable in considering the compliance of natural particles and complex biomolecules.
3. Mass Spectrometry: Mass spectrometry is utilized to decide the atomic mass and composition of a compound. It makes a difference in distinguishing obscure compounds, thinking about atomic parts, and analyzing biomolecules like proteins and peptides.
4. Infrared (IR) Spectroscopy: IR spectroscopy measures the retention of infrared light by atoms, giving data almost utilitarian bunches and chemical bonds displayed in a compound.
5. UV-Vis Spectroscopy: UV-Vis spectroscopy is utilized to consider the electronic moves and assimilation of light in atoms, advertising bits of knowledge into conjugation and chromophores.
6. Circular Dichroism (CD) Spectroscopy: CD spectroscopy could be a method utilized to ponder the auxiliary structure and chirality of biomolecules like proteins and nucleic acids.
7. Differential Scanning Calorimetry (DSC): DSC measures heat changes in a sample as it is subjected to controlled temperature variations, helping to study phase transitions, stability, and other thermal properties.
Computational Methods:
1. Molecular Mechanics (Force Fields): Molecular mechanics utilizes classical mechanics principles to simulate molecular structures and energy minimization, predicting molecular conformations and interactions.
2. Molecular Dynamics (MD) Simulations: MD reenactments recreate the movement and intelligence of molecules and particles over time, giving bits of knowledge into atomic behavior, conformational changes, and properties.
3. Quantum Mechanics (QM): Quantum mechanics strategies are utilized to unravel the Schrödinger condition, giving precise calculations of electronic structure, holding, and spectroscopic properties of atoms.
4. Density Functional Theory (DFT): DFT is a quantum mechanical method that approximates the electronic structure of molecules and materials, offering a balance between accuracy and computational cost.
5. Monte Carlo Simulations: Monte Carlo simulations use random sampling techniques to model molecular behavior, thermodynamics, and statistical mechanics of complex systems.
6. Computational Docking: Computational docking predicts the binding modes and interactions between molecules, assisting in drug design and understanding molecular recognition.
7. Ab Initio Methods: Ab initio methods solve the electronic structure of molecules from first principles, providing highly accurate results for small systems.
These test procedures and computational strategies are imperative devices for analysts in chemistry, organic chemistry, materials science, and other areas, empowering them to examine atomic frameworks, plan unused compounds, and unwind complex chemical wonders. Combining exploratory information with computational forecasts improves the understanding and revelation of chemical forms and materials.
Applications and Practical Implications
The understanding of conformation and configuration has various applications and common suggestions over different logical and mechanical areas.
Here are a few of the key applications and down-to-earth suggestions:
1. Drug Design and Pharmaceuticals:
• Knowledge of molecular conformation and configuration is crucial in drug design to optimize the bioactivity and pharmacological properties of pharmaceutical compounds.
• Configurational isomers, especially enantiomers, can exhibit different biological effects, and understanding their configuration is essential in designing safer and more effective drugs.
• A rational drug plan depends on computational strategies to anticipate and analyze atomic intelligence and conformational changes, supporting the advancement of novel therapeutics.
2. Material Science and Polymers:
• The conformation of molecules in materials significantly affects their physical and chemical properties, including mechanical strength, thermal stability, and optical activity.
• Understanding and controlling molecular conformation is fundamental in creating progressed materials for different applications, such as hardware, nanotechnology, and biomaterials.
3. Biochemistry and Molecular Biology:
• Protein conformation is critical in understanding enzyme function, protein-protein interactions, and molecular recognition processes in biological systems.
• The configuration of chiral molecules plays a key part in organic forms, such as the acknowledgment of biomolecules by receptors and proteins, which has suggestions for sedate advancement and cellular signaling.
4. Environmental Science:
• Understanding the conformation of pollutants and their interactions with the environment is essential for assessing environmental impact and designing effective remediation strategies.
5. Food Science and Flavor Chemistry:
• Conformation and configuration influence the flavor and aroma compounds in food products, affecting taste perception and sensory attributes.
• Identifying and controlling the configuration of key flavor molecules contributes to improving the taste and quality of food products.
6. Organic Chemistry and Synthesis:
• The conformational investigation is utilized to think about the reactivity and soundness of natural atoms, supporting the plan of modern engineered courses and the optimization of chemical forms.
7. Catalysis and Chemical Reactions:
• Understanding the conformational changes in catalysts and reactants is crucial in catalysis and chemical reactions, as different conformations can lead to different reaction pathways and selectivity.
8. Crystal Engineering and Supramolecular Chemistry:
• Controlling the conformation of molecules in crystal structures is important in crystal engineering to design materials with desired properties.
• Supramolecular chemistry involves the study of molecular assemblies and interactions based on conformational complementarity.
9. Pharmaceutical Formulation and Drug Delivery:
• Molecular conformation can influence the solubility and stability of drugs, affecting their formulation and delivery strategies.
The information on conformation and configuration has far-reaching applications and viable suggestions in the sedate plan, fabric science, natural chemistry, natural science, and other logical and mechanical spaces. It enables analysts and engineers to plan and create superior items, and progress forms, and pick up more profound bits of knowledge about atomic behavior and intuition.
Conclusion
Conformation and configuration are two fundamental concepts that govern the spatial arrangements of molecules and systems in various disciplines. Understanding their refinements is basic for investigators, analysts, engineers, and specialists working in ranges expanding from chemistry and science to computer science and texture science. Whether it’s anticipating sedate behavior, planning proficient frameworks, or investigating protein structures, the distinction between conformation and configuration plays a significant part in forming our understanding of the world around us.
