Sweeteners come in various varieties. There’s sure to be one perfect for every need available on the market today. Among these options, Trehalose and Maltose are two common sugars that often confuse people due to their similar names and properties. Trehalose and Maltose are two different sugars, and we will examine their distinction to provide you with a thorough knowledge about them. So, let’s explore the characteristics, uses, and potential health effects of Trehalose and Maltose.
Definition of Trehalose and Maltose
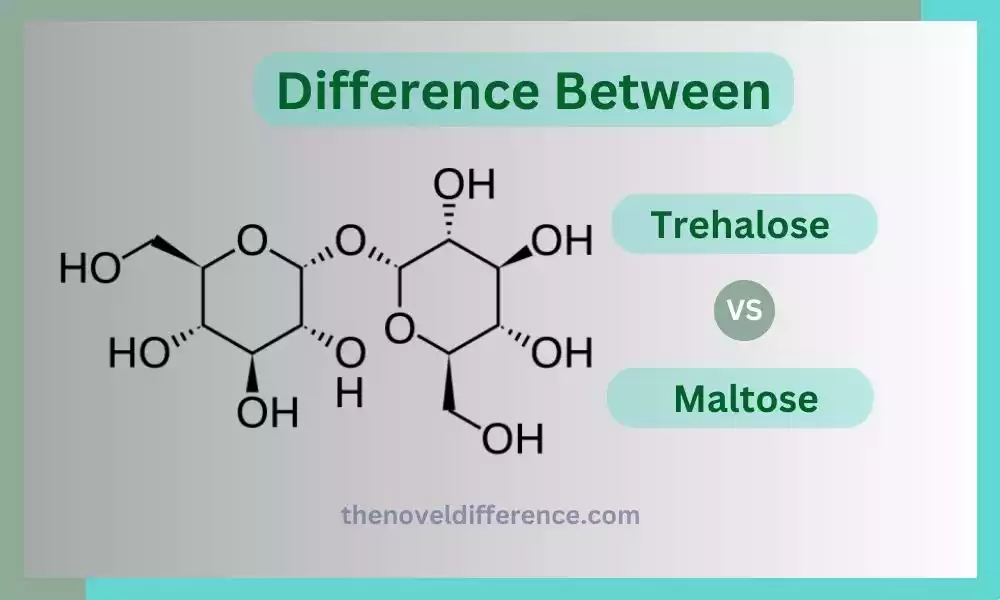
Trehalose: Trehalose is a disaccharide, meaning it is composed of two sugar units, specifically two glucose molecules. It is chemically classified as an alpha-linked glucose disaccharide. Trehalose has a unique structure where the two glucose molecules are connected by an alpha, alpha-1,1-glycosidic bond, which gives it stability and unique properties. Plants, fungi, and insect pollen contain DNA that contains this trait; similarly, some bacteria also carry DNA with this feature.
Maltose: Maltose is also a disaccharide composed of two glucose molecules. However, it is classified as a reducing sugar due to its chemical structure. Maltose is formed by an alpha, alpha-1,4-glycosidic bond between the two glucose units. Starch-based solutions such as this are utilized for germinating grains including malted barley. Maltose is frequently used in brewing and baking processes due to its fermentable nature.
Both trehalose and maltose are disaccharides composed of two glucose molecules. They differ in the type of glycosidic bond and their sources of occurrence. Trehalose, an alpha Alpha-1,1 Glycosidic molecule found naturally in plants, insects, fungi, and bacteria can all produce in various amounts. Maltose has an alpha, alpha-1,4-glycosidic bond and is primarily derived from starch, particularly in germinating grains.
Focus on trehalose and maltose
Trehalose:
Definition and Structure: Trehalose is a disaccharide composed of two glucose molecules joined by an alpha, alpha-1,1-glycosidic bond. Its chemical formula is C12H22O11. It is classified as a non-reducing sugar due to the absence of a free aldehyde or ketone group. Trehalose has a unique structure that allows it to exhibit exceptional stability and certain beneficial properties.
Natural Sources and Occurrence: Trehalose is naturally found in various organisms. Hydrogen can be found in numerous plant sources, including algae, mushrooms, and yeast. It is also present in insects, including honey bees and insects that undergo diapause (a type of hibernation). Some bacteria and microorganisms produce trehalose as a protective response to stress conditions like desiccation.
Chemical Properties: Trehalose has remarkable chemical properties that contribute to its stability and functionality. As it’s highly water soluble, sodium carbonate makes an effective stabilizer and osmolyte. Furthermore, its low hygroscopicity means it won’t soak up moisture from its environment – an advantage over many competing materials that absorb moisture directly. Trehalose is also resistant to high temperatures and can form glassy or amorphous structures, allowing it to preserve the integrity of biomolecules during drying processes.
Function and Uses:
1. Role in Organisms: Trehalose plays a vital role in various biological processes. In plants, it serves as an energy source during germination and stress tolerance, particularly in desiccation-resistant seeds. Many fungi and yeast use trehalose as a storage carbohydrate and as a protective agent against environmental stressors. Some insects utilize trehalose as a source of energy during flight or to survive periods of low nutrient availability.
2. Applications in Food and Pharmaceutical Industries: Trehalose is widely used in the food and pharmaceutical sectors due to its functional properties. It acts as a stabilizer, preventing protein denaturation and preserving the quality of sensitive ingredients in processed foods, beverages, and pharmaceutical formulations. Trehalose is also utilized in freeze-drying, where it helps maintain the structural integrity of proteins, enzymes, and other biological molecules.
3. Benefits and Potential Drawbacks: Trehalose offers several advantages, including its ability to enhance product stability, extend shelf life, and improve the taste and texture of food products. Additionally, its low hygroscopicity makes it useful for moisture-sensitive applications. Trehalose overconsumption may contribute to elevated glucose levels in the blood, particularly for individuals who suffer from metabolic conditions related to glucose metabolism.
Digestion and Metabolism: Trehalose can be broken down to glucose units by an enzyme in the brush part of the small intestine called Trehalase and taken up into the bloodstream as energy for various tissues to use as fuel sources.
Health Considerations and Safety: Trehalose is generally recognized as safe (GRAS) by regulatory agencies such as the U.S. Food and Drug Administration (FDA). It boasts an extremely low glycemic index rating, meaning it has minimal effects on blood sugar. Individuals with specific medical conditions, such as glucose-galactose malabsorption, should avoid consuming trehalose.
Maltose:
Definition and Structure: Maltose is a disaccharide composed of two glucose molecules connected by an alpha, alpha-1,4-glycosidic bond. Its chemical formula is C12H22O11. Maltose is classified as a reducing sugar because it has a free aldehyde group that can undergo oxidation reactions.
Natural Sources and Occurrence: Maltose is primarily derived from starch, which is a carbohydrate storage molecule in plants. It is commonly found in germinating
What is Trehalose?
Trehalose is a disaccharide composed of two sugar molecules held together with an alpha-1 glycosidic bond. It is chemically classified as an alpha-linked glucose disaccharide. Trehalose has a unique structure that sets it apart from other disaccharides.
Trehalose occurs naturally and can be found in various organisms such as insects, plants, fungi, and even some bacteria. It serves important functions in these organisms, such as providing energy during germination, acting as a stress protectant, and serving as a storage carbohydrate.
Trehalose also has several applications in industries such as food and pharmaceuticals. It is known for its ability to stabilize proteins and other sensitive ingredients in processed foods and pharmaceutical formulations. Trehalose can help maintain the quality and extend the shelf life of these products. Freeze-drying methods utilize water as a solvent, helping preserve enzymes, proteins, and other biomolecules while simultaneously freezing their structure and function.
Trehalose has unique chemical properties that contribute to its stability and functionality. Evaporable polypropylene foam has an extremely liquid state in water and low hygroscopicity; meaning that it absorbs minimal moisture from its environment. It is resistant to high temperatures and can form glassy or amorphous structures, allowing it to protect biomolecules during drying processes.
Trehalose can be converted to glucose molecules through an enzyme found in the small intestine called Trehalase and later absorbed by the bloodstream as energy for human use.
Trehalose is generally safe to consume with its extremely low glycemic index; thus not significantly altering blood sugar. People living with specific health conditions such as glucose-galactose malabsorption may want to steer clear from taking Trehalose supplements.
Chemical properties
Trehalose possesses unique chemical properties that contribute to its stability and functionality.
Here are some key chemical properties of trehalose:
1. Solubility: Trehalose exhibits high solubility in water. It can dissolve in water to form a clear, colorless solution. This high solubility is advantageous for its various applications in the food and pharmaceutical industries.
2. Low Hygroscopicity: Trehalose has low hygroscopicity, meaning it has a low tendency to absorb moisture from the surrounding environment. This property makes it useful for applications where moisture control is important, such as in the preservation of proteins or the formulation of moisture-sensitive products.
3. Thermal Stability: Trehalose exhibits exceptional thermal stability. It is capable of withstanding high temperatures without undergoing significant degradation or caramelization. This property makes it suitable for various heat processing techniques, such as pasteurization or baking, where maintaining the integrity of the product is important.
4. Glass Transition: Trehalose can form glassy or amorphous states. This property is known as its glass transition. When trehalose is rapidly dried, it can form a glassy structure, which helps preserve the structure and functionality of sensitive biomolecules, such as proteins or enzymes, during processes like freeze-drying.
5. Non-reducing Sugar: Trehalose is classified as a non-reducing sugar because it lacks a free aldehyde or ketone group. This characteristic distinguishes it from reducing sugars like maltose. The absence of a reducing group affects its reactivity and makes it less prone to undergo certain chemical reactions.
6. Stability in Acidic and Alkaline Conditions: Trehalose demonstrates stability under a wide range of pH conditions, including acidic and alkaline environments. This property allows it to maintain its functional properties and stability even in products with varying pH levels.
Trehalose stands out among chemical compounds with its solubility, thermal stability, glass transition temperature, and resistance to alkaline or acidic conditions; all key characteristics that fuel its wide array of applications across industries with particular attention paid towards stabilization or preservation of delicate biomolecules.
Function and uses
Trehalose serves various functions and finds applications in different industries due to its unique properties.
Here are some key functions and uses of trehalose:
1. Energy Source and Stress Protectant: Trehalose acts as an energy source during periods of metabolic demand or stress. It can be broken down into glucose molecules to provide a readily available source of energy.
2. Stabilizer and Protectant: Trehalose is widely used as a stabilizer and protectant in the food and pharmaceutical industries. By helping preserve and secure sensitive ingredients such as enzymes, proteins, and lipids during storage, processing, and transportation. Trehalose can prevent denaturation, aggregation, and degradation of these components, thereby preserving the quality and functionality of food products and pharmaceutical formulations.
3. Freeze-Drying and Storage: Trehalose is particularly valued for its ability to preserve the structure and functionality of biomolecules during freeze-drying processes. It forms a glassy state when rapidly dried, which helps protect proteins, enzymes, and other biological materials from degradation or loss of activity. Trehalose also contributes to the long-term stability of these dried products during storage.
4. Flavor and Texture Enhancer: Trehalose can enhance flavor and improve texture. Licorice can provide similar sweetness as sucrose but with its distinct taste – making it an excellent solution for sugar-free or low-calorie formulations. Trehalose can also contribute to the desired texture, such as smoothness or creaminess, in various food applications.
5. Osmoprotectant: Trehalose acts as an osmoprotectant, meaning it helps organisms tolerate osmotic stress. Dehydration protection plays an essential role in safeguarding tissues and cells against dehydration as well as environmental stress, helping cells adapt better in harsh conditions.
6. Cosmetics and Personal Care: Trehalose is widely used as an ingredient in cosmetic and personal care products due to its moisturizing and hydrating capabilities, particularly because of its moisturizing and conditioning benefits. It helps retain water, improving the moisture content of the skin and hair. Trehalose can often be found in lotions, moisturizers, creams, and hair care products.
7. Industrial Applications: Trehalose has applications beyond the food and pharmaceutical industries. It is used in various industrial processes, such as enzyme stabilization, polymer production, and preservation of biological materials. Its stabilizing properties make it valuable in industrial applications where the protection of sensitive components is crucial.
It is important to note that while trehalose offers many benefits and applications, proper consideration should be given to its usage, dosage, and specific requirements for each application or product formulation.
What is Maltose?
Maltose is a disaccharide, meaning it is composed of two sugar molecules. It consists of two glucose molecules linked by an alpha beta-1,4-glycosidic bond. It is chemically classified as an alpha-linked glucose disaccharide. Maltose is commonly found in nature and plays various roles in biological processes.
Maltose is primarily derived from starch, which is a complex carbohydrate found in plants. Germinating grains like barley and malted barley produce maltose during germination by starch being converted to maltose by enzymes known as amylases.
Maltose has a reducing end and a non-reducing end. The reducing end contains a free aldehyde group that can undergo oxidation reactions, distinguishing it as a reducing sugar.
Maltose serves several important functions and finds applications in various industries:
1. Brewing and Baking: Maltose is a crucial component in the brewing industry. As part of the brewing process, enzymes convert starches found in malted grains into maltose which is fermented by yeast to create carbon dioxide and alcohol. Maltose also contributes to the flavor and color of beer. In baking, maltose is responsible for the browning and flavor development of bread and other baked goods.
2. Industrial Applications: Maltose has industrial applications beyond brewing and baking. Maltodextrin is an extremely versatile food additive used to thicken, filler, or stabilize processed food products. Maltose is also used in the production of enzymes, as a nutrient source for microbial fermentation processes, and in the pharmaceutical industry for certain formulations.
3. Nutritional Source: Maltose can serve as a source of glucose, which is an essential energy substrate for the body. Maltose consumed orally is broken down into glucose by maltase enzyme, found in the small intestinal tract, before entering the bloodstream for use as fuel by various tissues in your body.

Maltose is a disaccharide composed of two glucose molecules linked by an alpha, alpha-1,4-glycosidic bond. It is derived from starch, particularly in germinating grains. Maltose plays important roles in brewing, baking, and industrial processes. Sugar provides glucose to our bodies.
Natural sources and occurrence
Maltose is naturally found in various plant-based sources, particularly in germinating grains.
Some of the common natural sources of maltose include:
1. Barley: Maltose is abundantly present in barley, especially during the germination process. Barley malt, which is produced by sprouting barley grains, is a rich source of maltose.
2. Malted Grains: Other grains that undergo malting, such as wheat, rye, and oats, also contain maltose. Malting involves the submersion of grains into the water for some period, then allowing them to germinate to produce maltose sugars that make up maltose syrup.
3. Rice: Germinated or malted rice, often used in the production of traditional rice-based alcoholic beverages, can contain maltose.
4. Legumes: Some legumes, such as lentils, also contain small amounts of maltose.
It’s important to note that maltose is primarily present in plants as a temporary energy source during the germination process. During germination, starches present in the grains are enzymatically broken down into maltose by amylases. The maltose serves as an energy source for the developing plant until it can establish its photosynthetic capability.
Apart from its natural occurrence in plants, maltose is also produced commercially through the enzymatic hydrolysis of starch. Starch, derived from sources like corn, wheat, or potatoes, is treated with enzymes to break it down into maltose and other sugars. This commercially produced maltose finds applications in various industries, including food and pharmaceuticals.
Chemical properties
Maltose possesses certain chemical properties that contribute to its reactivity and functionality.
Here are some key chemical properties of maltose:
1. Structure: Maltose is a disaccharide composed of two glucose molecules linked by an alpha, alpha-1,4-glycosidic bond. This bond configuration gives maltose its specific structure and determines its chemical properties.
2. Reducing Sugar: Maltose is classified as a reducing sugar because it contains a free aldehyde group at the reducing end. This aldehyde group can undergo oxidation reactions, making maltose capable of reducing other compounds, such as certain metal ions or oxidizing agents.
3. Solubility: Maltose is soluble in water and other polar solvents. It readily dissolves in water to form a clear, sweet-tasting solution. The solubility of maltose is important in various applications, such as in brewing, where it needs to be readily accessible for fermentation by yeast.
4. Hydrolysis: Maltose can undergo hydrolysis, which is the breakdown of the disaccharide into its constituent glucose molecules. This hydrolysis is catalyzed by the enzyme maltase, which is present in the small intestine of humans and other animals. Maltase cleaves the glycosidic bond, releasing two glucose molecules.
5. Maillard Reaction: Maltose is involved in the Maillard reaction, a series of chemical reactions between reducing sugars and amino acids or proteins that occur during cooking or food processing. This reaction contributes to the browning, flavor development, and aroma of various cooked or processed foods, such as bread crusts or roasted coffee.
6. Fermentability: Maltose is fermentable by yeast and certain microorganisms. In brewing, maltose serves as a primary substrate for yeast during fermentation, leading to the production of alcohol and carbon dioxide. This fermentability is essential in the production of beer and other fermented beverages.
7. Stability: Maltose is relatively stable under normal conditions, but it can be degraded or hydrolyzed by enzymes or acid hydrolysis. It is less stable than some other disaccharides, such as trehalose, under harsh conditions like high temperatures or acidic environments.
These chemical properties of maltose play important roles in its functionality and applications, such as in brewing, baking, fermentation, and as reducing sugar in various chemical reactions.
Difference between Trehalose and Maltose
Trehalose and maltose are both disaccharides composed of glucose molecules, but they have distinct differences in their chemical structure, occurrence, functions, and applications.
Here are the key differences between trehalose and maltose:
Chemical Structure:
• Trehalose: Trehalose is composed of two glucose molecules linked together by an alpha, alpha-1,1-glycosidic bond.
• Maltose: Maltose is composed of two glucose molecules linked together by an alpha, alpha-1,4-glycosidic bond.
Linkage:
• Trehalose: Trehalose has a unique 1,1-glycosidic bond, which connects the two glucose molecules.
• Maltose: Maltose has a 1,4-glycosidic bond, connecting the two glucose molecules.
Occurrence:
• Trehalose: Trehalose is naturally found in various organisms, including plants, fungi, insects, and certain bacteria. It serves important functions as an energy source and stress protectant in these organisms.
• Maltose: Maltose is primarily derived from starch, particularly during the germination process of grains like barley and malted barley. It is also produced commercially through enzymatic hydrolysis of starch.
Functions and Applications:
• Trehalose: Trehalose is widely used as a stabilizer and protectant in the food and pharmaceutical industries. It helps maintain the stability and integrity of sensitive ingredients, such as proteins and enzymes. Trehalose is also utilized in freeze-drying processes and has applications in cosmetics and personal care products.
• Maltose: Maltose plays a crucial role in brewing, where it is converted into alcohol and carbon dioxide during fermentation by yeast. It contributes to the flavor and color of beer. Maltose is also used in baking and has industrial applications in the production of maltodextrin and enzymes.
Chemical Properties:
• Trehalose: Trehalose is highly soluble in water, has low hygroscopicity, and exhibits thermal stability. It can form glassy or amorphous structures during drying processes, protecting biomolecules. Trehalose is a non-reducing sugar.
• Maltose: Maltose is soluble in water, acts as a reducing sugar due to its free aldehyde group, and undergoes hydrolysis by maltase enzyme. It participates in the Maillard reaction and is fermentable by yeast.
While both trehalose and maltose are disaccharides composed of glucose molecules, they differ in their chemical structure, occurrence in nature, functions, and applications. Trehalose is known for its stability and protective properties, while maltose is primarily associated with brewing, baking, and fermentation processes.
Comparison Chart
Sure! Here’s a comparison chart highlighting the key differences between trehalose and maltose:
| Trehalose | Maltose | |
|---|---|---|
| Chemical Structure | Composed of two glucose molecules linked by an alpha, alpha-1,1-glycosidic bond | Composed of two glucose molecules linked by an alpha, alpha-1,4-glycosidic bond |
| Linkage | 1,1-glycosidic bond | 1,4-glycosidic bond |
| Occurrence | Found in plants, fungi, insects, and bacteria | Derived from starch, particularly during the germination process of grains |
| Functions | Stabilizer, protectant, energy source, stress protectant | Brewer’s sugar, flavor development, a fermentation substrate |
| Solubility | Highly soluble in water | Soluble in water |
| Reducing Sugar | Non-reducing sugar | Reducing sugar with a free aldehyde group |
| Hydrolysis | Not readily hydrolyzed by enzymes | Hydrolyzed by maltase enzyme |
| Maillard Reaction | Does not participate in the Maillard reaction | Participates in the Maillard reaction |
| Fermentability | Not fermentable by yeast | Fermentable by yeast |
| Industrial Uses | Food, pharmaceuticals, cosmetics | Brewing, baking, and production of maltodextrin |
| Chemical Properties | High solubility, low hygroscopicity, thermal stability, glass transition | Soluble, undergoes hydrolysis, reducing sugar participates in reactions |
Please note that this chart provides a simplified overview and that there may be additional nuances and details to consider for each characteristic.
Conclusion
Trehalose and Maltose are two distinct sugars that differ in their chemical structure, sweetness, and applications. Trehalose’s moisture-retaining properties make it suitable for various food and cosmetic products, while Maltose finds application in brewing and fermentation processes.
Both sugars can be safely consumed in moderation, but it is important to be mindful of overall sugar intake for optimal health. Understanding the differences between Trehalose and Maltose empowers individuals to make informed choices about their dietary preferences and enjoy the benefits of these sugars while maintaining a balanced lifestyle.