Lactide and lactone are two terms often encountered in the field of chemistry, particularly about organic compounds. Whereas they may sound comparable, they have particular contrasts that set them separated. We are going investigate the dissimilarities between lactide and lactone and pick up a more profound understanding of their special properties and applications.
Introduction
When delving into the realm of organic chemistry, it is crucial to grasp the disparities between various compounds to accurately comprehend their behaviors and functions. Lactide and lactone are two such compounds that share some similarities but are fundamentally different. Let’s jump into the complexities of these substances and shed light on their dissimilarities.
Importance and applications of lactide and lactone
Lactide and lactone are important chemical compounds with various applications in different industries.
Here are some of their significant uses:
1. Lactide:
a. Generation of Polylactic Corrosive (PLA): Lactide may be a key middle within the generation of polylactic corrosive, a biodegradable and renewable polymer. PLA finds applications in packaging materials, disposable cutlery, textiles, biomedical devices, and more. Lactide serves as a monomer that undergoes polymerization to form PLA.
b. Medical and Pharmaceutical Applications: Lactide-based polymers have gained attention in the medical field. They are used in the development of drug delivery systems, tissue engineering scaffolds, surgical sutures, and bioresorbable implants. Lactide’s biocompatibility and controllable degradation properties make it suitable for biomedical applications.
c. Cosmetics and Personal Care Products: Lactide derivatives, such as lactide-based copolymers or oligomers, are used in cosmetic and personal care formulations. They can enhance the stability, texture, and sensory properties of products like creams, lotions, and hair care items.
d. Packaging and Films: Lactide-based polymers are employed in packaging films due to their biodegradability and barrier properties. They help prolong the shelf life of food products while reducing environmental impact.
e. Agricultural and Horticultural Applications: Lactide-based materials find use in agricultural applications, such as controlled-release fertilizers and biodegradable mulch films. These applications provide sustainable alternatives to conventional non-biodegradable materials.
2. Lactone:
a. Flavor and Fragrance Compounds: Lactones contribute to the flavor and aroma profiles of many natural products. They are commonly found in natural products like peaches, pineapples, and strawberries, including sweet and fruity notes. Lactones are utilized within the generation of fake flavorings and scents for nourishment, refreshments, aromas, and makeup.
b. Polymer Synthesis: Lactones serve as monomers in the synthesis of various polymers. ε-caprolactone is utilized to create polycaprolactone, which finds applications in therapeutic gadgets, sedate conveyance frameworks, and biodegradable plastics. Other lactones, such as γ-butyrolactone and δ-valerolactone, are moreover utilized in polymer blends.
c. Solvents and Cleaning Agents: Some lactones, like γ-butyrolactone, are used as solvents for various chemical reactions and as cleaning agents. They possess excellent solvency properties and are employed in industrial applications, such as paint and coating formulations, polymer processing, and electronics manufacturing.
Lactide and lactone play crucial roles in industries ranging from plastics and packaging to pharmaceuticals and flavors. Their unique properties make them versatile compounds with wide-ranging applications, contributing to sustainable and innovative solutions in various sectors.
Definition and Structure
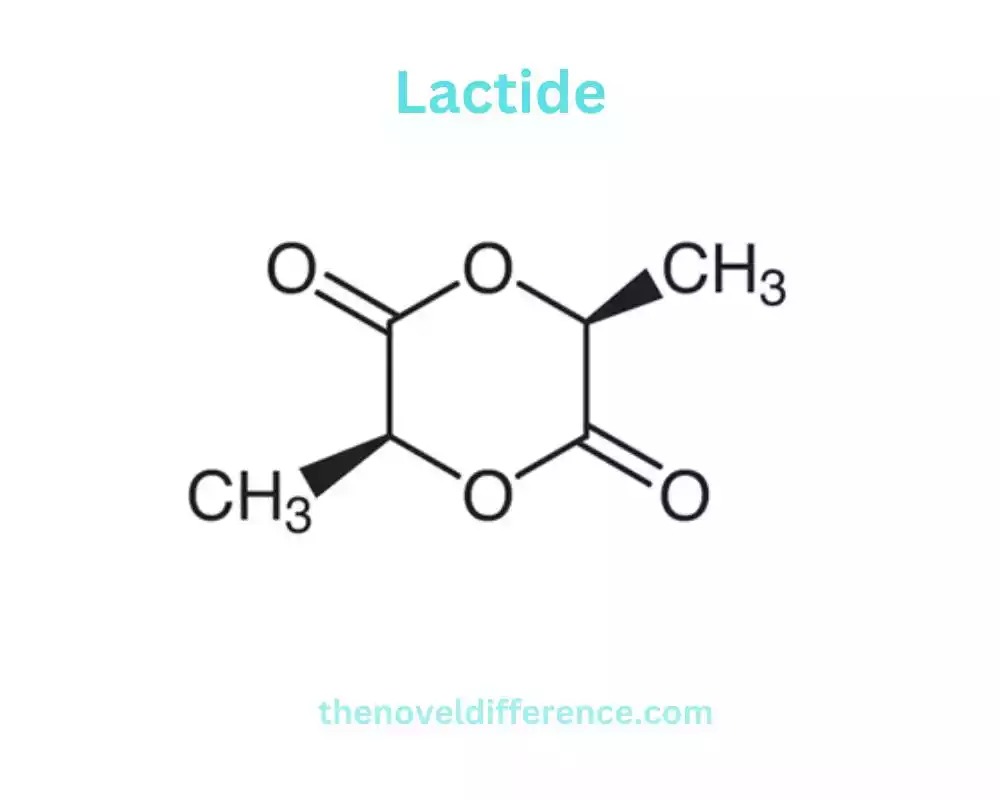
Lactide: Lactide is a cyclic diester of lactic acid. It is shaped by the condensation of two particles of lactic corrosive, coming about within the end of a water atom. Lactide exists as a dimer and has a cyclic structure. It is commonly represented as a ring structure composed of two ester functional groups connected by a carbon-carbon bond. Lactides can exist in totally different stereochemical shapes, counting L-lactide, D-lactide, and meso-lactide, depending on the arrangement of substituents around the carbon particles within the ring. L-lactide and D-lactide are enantiomers, whereas meso-lactide could be a diastereomer.
Lactone: Lactone may be a cyclic ester determined from a hydroxy corrosive. It is characterized by the nearness of a cyclic ester useful gather, which shapes when a hydroxyl gather (-Gracious) of a natural corrosive responds with a carboxylic corrosive group (-COOH) inside the same particle, coming about within the disposal of a water particle. Lactones can vary in ring size, with the most common being five- or six-membered rings. They are named based on the number of carbon particles within the ring, such as gamma-lactone (5-membered ring) or delta-lactone (6-membered ring). Lactones can also have different substituents attached to the ring, giving rise to various types of lactones with different chemical and physical properties.
What is Lactide?
Lactide is a cyclic diester of lactic acid, a compound commonly found in nature. It is shaped through the condensation response of two atoms of lactic corrosive, coming about within the disposal of a water atom. Lactide could be a key middle within the generation of polylactic corrosive (PLA), a biodegradable and renewable polymer.
Lactide exists as a dimer and has a cyclic structure. It is typically represented as a ring structure composed of two ester functional groups connected by a carbon-carbon bond. The structure of lactide can vary depending on the stereochemistry of the molecule. It can exist in several shapes, counting L-lactide, D-lactide, and meso-lactide.
L-lactide and D-lactide are enantiomers, which suggests they are non-superimposable and reflect pictures of each other. These enantiomers have distinct stereochemical properties and can exhibit different reactivities and characteristics in polymerization processes. Meso-lactide, on the other hand, is a diastereomer and lacks chiral properties.
Lactide is of critical significance due to its part in the generation of polylactic acid (PLA). PLA may be a flexible polymer utilized in different applications, counting bundling materials, expendable cutlery, materials, biomedical gadgets, and more. Lactide serves as a monomer that undergoes polymerization to form PLA, which has gained attention as a sustainable and eco-friendly alternative to traditional plastics.
Process of lactide formation
The formation of lactide involves the condensation reaction of lactic acid molecules. The process can occur through various methods, including thermal polymerization and chemical catalysts.
Here is a general overview of the process:
1. Thermal Polymerization:
a. Lactic Acid Preparation: Lactic corrosive, a carboxylic corrosive, is ordinarily gotten through the aging of carbohydrates by microbes or parasites. It can be delivered from renewable assets such as corn or sugarcane.
b. Heating and Dehydration: Lactic acid is subjected to heat in the presence of a dehydration agent, such as azeotropic distillation or vacuum conditions. The heat and reduced pressure help remove water from the reaction mixture.
c. Lactide Formation: As water is eliminated, two molecules of lactic acid condense, resulting in the formation of lactide. The response continues through the end of a water particle, with the carboxylic corrosive bunch of one lactic corrosive particle responding with the hydroxyl gathers of another lactic corrosive atom. This leads to the formation of a cyclic diester, lactide.
d. Lactide Isolation: The lactide formed is then purified by various methods, such as distillation, crystallization, or chromatography, to separate it from any remaining lactic acid or impurities. This step helps obtain pure lactide for further processing or polymerization.
2. Chemical Catalysts: The formation of lactide can be facilitated by chemical catalysts, which accelerate the reaction and improve selectivity. Common catalysts used for lactide formation include metal-based compounds, such as tin(II) alkoxides or tin(II) carboxylates. These catalysts promote the esterification and dehydration reactions between lactic acid molecules to form lactide.
The specific conditions, reaction temperatures, catalysts, and purification methods may vary depending on the desired outcome and the scale of production. The formation of lactide is a crucial step in the production of polylactic acid (PLA), where lactide monomers further undergo polymerization to form the final polymer structure.
Common methods for synthesizing lactide
There are several common methods for synthesizing lactide. Here are three commonly used approaches:
1. Direct Esterification:
a. Lactic Acid Preparation: Lactic acid, the starting material, is typically obtained through fermentation processes or chemical synthesis from renewable resources.
b. Acid-Catalyzed Esterification: Lactic acid is reacted with an acid catalyst, such as sulfuric acid or p-toluenesulfonic acid. The corrosive catalyst makes a difference encourage the esterification response between the carboxylic corrosive gather of lactic corrosive and the hydroxyl gather of another lactic corrosive atom.
c. Dehydration: The response blend is subjected to raised temperatures, regularly within the extent of 150-200°C. The heat promotes the elimination of water from the reaction, driving the formation of lactide. This step involves the condensation of two lactic acid molecules to form a cyclic diester lactide.
d. Lactide Purification: The lactide formed is isolated and purified using techniques such as distillation, crystallization, or chromatography to obtain pure lactide for further processing or polymerization.
2. Ring-Opening Polymerization:
a. Lactide Monomer Preparation: Lactic acid is first converted into lactide through esterification, as described in the direct esterification method or other suitable methods.
b. Polymerization Catalyst: A catalyst, such as a metal-based compound (e.g., tin(II) alkoxides), is added to initiate the ring-opening polymerization of lactide. The catalyst helps break the cyclic structure of lactide and facilitates the formation of polymer chains.
c. Polymerization Reaction: The lactide monomers undergo ring-opening polymerization, where the cyclic ester bonds of lactide open, and the monomers join together to form a polymer chain. This process occurs through the coordination of the catalyst with the lactide monomer.
d. Polymerization Control: The reaction conditions, such as temperature, catalyst concentration, and reaction time, are carefully controlled to achieve the desired molecular weight and properties of the resulting polymer.
3. Enzymatic Polymerization:
a. Lactic Acid Conversion: Lactic acid is enzymatically converted into lactide using specific enzymes, such as lipases or immobilized lactic acid bacteria.
b. Enzymatic Transesterification: The lactic corrosive is subjected to enzymatic transesterification, where the protein encourages the esterification response between the carboxylic corrosive gather of lactic corrosive and the hydroxyl bunch of another lactic corrosive atom.
c. Lactide Formation: The enzymatic transesterification reaction leads to the formation of lactide as the esterification proceeds, resulting in the condensation of two lactic acid molecules.
d. Lactide Isolation and Purification: The lactide formed is isolated and purified using suitable techniques to obtain pure lactide for subsequent processing or polymerization.
These methods provide different approaches to synthesizing lactide, allowing for the production of lactide on various scales and different applications, particularly in the synthesis of polylactic acid (PLA). The choice of method depends on factors such as desired purity, process efficiency, and specific requirements of the application.
What is Lactone?
Lactone could be a cyclic ester, which could be a sort of natural compound. It is shaped through the intramolecular esterification response between a carboxylic corrosive bunch (-COOH) and a hydroxyl bunch (-Goodness) inside the same particle. This reaction leads to the formation of a cyclic structure with a carbonyl group (C=O) and an oxygen atom within the ring.
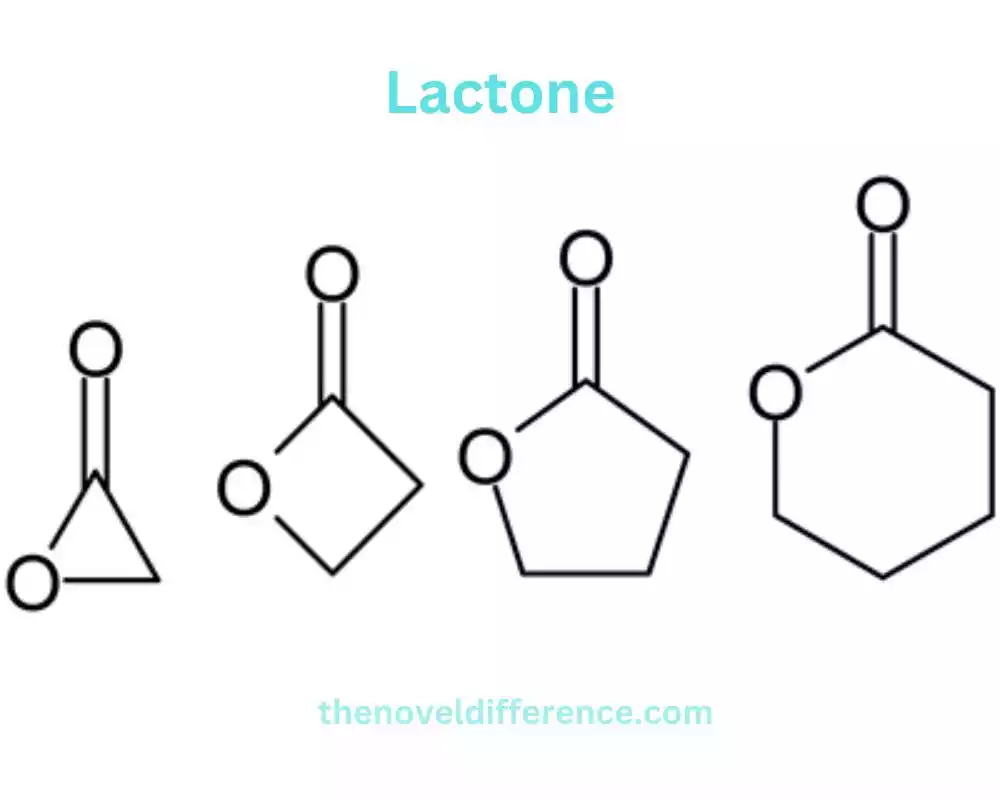
Lactones are named based on the number of carbon atoms in the ring and are often denoted by Greek letters.
For example:
• γ-lactone: a five-membered ring lactone
• δ-lactone: a six-membered ring lactone
• ε-lactone: a seven-membered ring lactone
The common structure of lactones comprises a carbonyl gather (C=O) reinforced to one carbon molecule inside the ring and an oxygen molecule reinforced to another carbon molecule inside the ring. The remaining carbon atoms complete the ring structure.
Lactones can have varying substituents attached to the carbon atoms of the ring, leading to different types of lactones with distinct chemical properties and reactivities. The presence of the cyclic ester group gives lactones unique characteristics, including the ability to undergo hydrolysis reactions, and ring-opening reactions, and participate in various chemical transformations.
Lactones are found normally in different sources, counting natural products, where they contribute to the characteristic flavors and smells. They are too synthesized for utilization as flavor and scent compounds within the nourishment, refreshment, fragrance, and beauty care products businesses. Also, lactones have applications in polymer blends, where they serve as monomers or building pieces for the generation of different polymers, such as polycaprolactone.
Lactones are important compounds with diverse applications and play a significant role in the flavor, fragrance, and polymer industries.
Process of lactone formation
The arrangement of lactones includes the intramolecular esterification response between a carboxylic corrosive gather (-COOH) and a hydroxyl gather (-Goodness) inside the same particle. The process can occur through different methods, including acid-catalyzed or base-catalyzed reactions.
Here’s an overview of the process:
1. Acid-Catalyzed Lactone Formation:
a. Carboxylic Acid Preparation: The beginning fabric may be a carboxylic corrosive, which can be gotten from different sources, such as natural amalgamation or common items.
b. Acid Catalysis: The carboxylic corrosive is subjected to a corrosive catalyst, such as sulfuric corrosive or p-toluene sulfonic corrosive. The acid catalyst assists in protonating the carboxylic acid group, making it more reactive toward the intramolecular esterification reaction.
c. Intramolecular Esterification: The acid-catalyzed response happens when the protonated carboxylic corrosive gather responds with the adjoining hydroxyl gather inside the same particle. This results in the formation of a cyclic ester bond, forming the lactone.
d. Lactone Isolation and Purification: The lactone formed is isolated and purified using techniques such as extraction, distillation, or chromatography to obtain pure lactone for further use or characterization.
2. Base-Catalyzed Lactone Formation:
a. Carboxylic Acid Preparation: Similar to the acid-catalyzed method, the starting material is a carboxylic acid obtained from appropriate sources.
b. Base Catalysis: The carboxylic corrosive is treated with a base catalyst, such as sodium hydroxide or potassium carbonate. The base catalyst helps activate the carboxylic acid group, making it more susceptible to nucleophilic attack by the adjacent hydroxyl group.
c. Intramolecular Esterification: The actuated carboxylic corrosive bunch responds with the adjoining hydroxyl bunch inside the same particle, driving the arrangement of a cyclic ester bond and the era of the lactone.
d. Lactone Isolation and Purification: Similar to the acid-catalyzed method, the lactone formed is isolated and purified using suitable techniques to obtain pure lactone for further use or characterization.
The specific reaction conditions, such as temperature, catalyst concentration, and reaction time, can influence the efficiency and selectivity of lactone formation. The choice between acid-catalyzed or base-catalyzed strategies depends on components such as the reactivity of the beginning materials and craved response conditions.
Lactone formation is an important process in various fields, including organic synthesis, the flavor and fragrance industry, and polymer chemistry, where lactones serve as important building blocks for the synthesis of diverse compounds and materials.
Common methods for synthesizing lactone
There are several common methods for synthesizing lactones. Here are three commonly used approaches:
1. Intramolecular Cyclization:
a. Carboxylic Acid Preparation: The beginning fabric may be a carboxylic corrosive, which can be gotten through natural amalgamation or confined from normal sources.
b. Activation of Carboxylic Acid: The carboxylic corrosive is enacted through the expansion of a reagent or a combination of reagents, such as acyl chlorides (e.g., thionyl chloride), corrosive anhydrides, or carbodiimides. This step facilitates the formation of a more reactive acyl intermediate.
c. Intramolecular Cyclization: The actuated carboxylic corrosive experiences intramolecular cyclization, where the acyl middle responds with an adjoining hydroxyl bunch inside the same particle. This reaction forms the cyclic ester bond, resulting in the formation of the desired lactone.
d. Lactone Isolation and Purification: The lactone formed is isolated and purified using techniques such as extraction, distillation, or chromatography to obtain pure lactone for further use or characterization.
2. Ring-Closing Metathesis:
a. Olefinic Precursor Preparation: The starting material is an olefinic compound containing a terminal alkene or internal alkene, which will serve as the precursor for lactone formation.
b. Metathesis Catalyst: A suitable metathesis catalyst, such as a ruthenium-based catalyst (e.g., Grubbs catalyst), is added to facilitate the ring-closing metathesis reaction.
c. Ring-Closing Metathesis: The metathesis catalyst promotes the reaction between the olefinic precursor, resulting in the formation of a cyclic intermediate. This intermediate then undergoes subsequent reactions, such as tautomerization or hydrogenation, to form the desired lactone.
d. Lactone Isolation and Purification: The lactone formed is isolated and purified using appropriate techniques to obtain pure lactone for further use or characterization.
3. Oxidative Lactonization:
a. Alcohol or Aldehyde Precursor: The starting material is an alcohol or aldehyde compound containing a suitable functional group for lactonization.
b. Oxidation: The alcohol or aldehyde precursor is subjected to oxidation conditions, often using an oxidizing agent such as a hypervalent iodine reagent (e.g., Dess-Martin periodinane) or a metal-based oxidant (e.g., silver or palladium catalysts).
c. Lactonization: The oxidation reaction induces intramolecular cyclization, leading to the formation of the desired lactone by incorporating the alcohol or aldehyde moiety into the ring structure.
d. Lactone Isolation and Purification: The lactone formed is isolated and purified using appropriate techniques to obtain pure lactone for further use or characterization.
These methods provide different approaches to synthesizing lactones, allowing for the production of diverse lactone structures and functional groups. The choice of method depends on factors such as the availability of starting materials, desired reaction conditions, and the specific lactone structure required for a particular application.
Differences Between Lactide and Lactone
Lactide and lactone are two distinct compounds with different structures and properties.
Here are the key differences between lactide and lactone:
1. Structure:
• Lactide: Lactide is a cyclic diester of lactic acid, consisting of two lactic acid molecules condensed together. It has a cyclic structure with two ester functional groups connected by a carbon-carbon bond. Lactides can exist in numerous shapes, counting L-lactide, D-lactide, and meso-lactide.
• Lactone: Lactone is a cyclic ester, formed through the intramolecular esterification reaction within a single molecule. It contains a carbonyl group (C=O) and an oxygen atom within the ring. Lactones can have distinctive ring sizes and are named based on the number of carbon particles within the ring, such as γ-lactone, δ-lactone, and ε-lactone.
2. Formation:
• Lactide: Lactide is typically formed through the condensation reaction of two molecules of lactic acid, resulting in the elimination of a water molecule. This process is known as esterification or dehydration of lactic acid.
• Lactone: Lactone formation involves the intramolecular esterification reaction between a carboxylic acid group and a hydroxyl group within the same molecule. This reaction leads to the formation of a cyclic ester bond and the generation of the lactone.
3. Occurrence:
• Lactide: Lactide is an intermediate compound in the production of polylactic acid (PLA). It is not commonly found in nature but is synthesized from lactic acid, which can be derived from renewable resources or produced through fermentation processes.
• Lactone: Lactones are naturally occurring compounds found in various sources, including fruits, where they contribute to the characteristic flavors and aromas. Lactones are too synthesized for utilization as flavor and scent compounds within the nourishment, refreshment, fragrance, and beauty care products businesses.
4. Applications:
• Lactide: Lactide is fundamentally utilized as a monomer within the generation of polylactic corrosive (PLA), a biodegradable and renewable polymer. PLA includes a wide extend of applications, counting bundling materials, expendable cutlery, materials, biomedical gadgets, and more.
• Lactone: Lactones have diverse applications in industries such as food, fragrance, and pharmaceuticals. They are used as flavor and fragrance compounds, contributing to the aroma and taste of various products. Lactones are also employed as intermediates in organic synthesis and serve as building blocks for the synthesis of other compounds.
Lactide and lactone differ in their molecular structures, formation processes, occurrence in nature, and applications. Lactide is a cyclic diester of lactic acid and is primarily used in the production of PLA, while lactone is a cyclic ester that occurs naturally and finds applications in flavors, fragrances, and organic synthesis.
Comparison Chart
Sure! Here’s a comparison chart highlighting the key differences between lactide and lactone:
| Lactide | Lactone | |
|---|---|---|
| Structure | Cyclic diester of lactic acid, consisting of two lactic acid molecules condensed together | Cyclic ester is formed through an intramolecular esterification reaction within a single molecule |
| Formation | Condensation of two molecules of lactic acid, eliminating a water molecule | Intramolecular esterification reaction between carboxylic acid and hydroxyl group within the same molecule |
| Occurrence | Not commonly found in nature; synthesized from lactic acid derived from renewable resources or fermentation | Naturally occurring compounds found in various sources, including fruits |
| Applications | Primarily used as a monomer in the production of polylactic acid (PLA) | Used as flavor and fragrance compounds, intermediates in organic synthesis, and building blocks for other compounds |
| Examples | L-lactide, D-lactide, meso-lactide | γ-lactone, δ-lactone, ε-lactone |
| Industrial Use | A key component in the production of biodegradable and renewable PLA polymer | Flavor and fragrance industry, organic synthesis |
| Ring Size | No specific ring size depends on the lactic acid molecule used | Varies based on the number of carbon atoms in the ring (e.g., γ-lactone has a five-membered ring) |
This chart summarizes the main differences between lactide and lactone, including their structures, formation processes, occurrence, applications, and examples.
Applications and Properties
The applications and properties of lactide and lactone are as follows:
Applications of Lactide:
1. Polylactic Acid (PLA) Production: Lactide is a crucial monomer used in the production of polylactic acid (PLA), a biodegradable and renewable polymer. PLA contains a wide run of applications, counting bundling materials, expendable cutlery, materials, biomedical gadgets, and more.
2. Biomedical Applications: Lactide-based polymers, such as poly(lactic-co-glycolic corrosive) (PLGA), are broadly utilized in biomedical applications, counting sedate conveyance frameworks, tissue designing, and frameworks for regenerative medication.
Properties of Lactide:
1. Biodegradability: Lactide-based polymers, such as PLA, are biodegradable, meaning they can be broken down by natural processes over time. This property is beneficial for reducing environmental impact and promoting sustainability.
2. Thermal Stability: Lactide-based polymers exhibit good thermal stability, enabling them to withstand various processing conditions, such as melt processing and sterilization.
3. Mechanical Properties: The mechanical properties of lactide-based polymers can be tailored by adjusting the ratio of L-lactide and D-lactide monomers. This permits the generation of PLA with a wide run of solidness, quality, and adaptability appropriate for diverse applications.
Applications of Lactone:
1. Flavor and Fragrance Industry: Lactones are widely used as flavor and fragrance compounds in the food, beverage, perfume, and cosmetics industries. They contribute to the characteristic flavors and smells of different items, counting natural products, dairy items, and alcoholic refreshments.
2. Organic Synthesis: Lactones serve as important intermediates in organic synthesis. They are utilized within the generation of different compounds, such as pharmaceuticals, agrochemicals, and strength chemicals.
3. Polymer Chemistry: Certain lactones, such as ε-caprolactone, are utilized in polymer chemistry as monomers or building blocks for the synthesis of biodegradable polymers, including polycaprolactone (PCL). These polymers discover applications in sedate conveyance frameworks, tissue building, and 3D printing.
Properties of Lactone:
1. Volatility and Aroma: Lactones possess characteristic flavors and aromas, contributing to the sensory profiles of different products. The specific odor and taste properties can vary depending on the lactone structure and the presence of substituents.
2. Ring Size Influence: The size of the lactone ring can affect its physical and chemical properties. For example, smaller ring lactones, such as γ-lactones, tend to have higher volatility and stronger aroma compared to larger ring lactones.
3. Reactivity: Lactones can undergo various chemical transformations, including hydrolysis, ring-opening reactions, and functional group modifications. This reactivity allows for their utilization in diverse synthetic pathways and applications in organic chemistry.
Lactide and lactone have distinct applications and properties. Lactide finds primary use in the production of PLA, while lactone is utilized in the flavor and fragrance industry, organic synthesis, and polymer chemistry. Understanding these applications and properties enables their efficient utilization in various fields.
Conclusion
Lactide and lactone may share similarities due to their cyclic ester nature, but they possess distinct differences in terms of their chemical structures, formation processes, applications, and reactivity. Understanding these contrasts is vital for researchers, chemists, and analysts working with these compounds to saddle their one-of-a-kind properties for different mechanical and logical endeavors. By delving into the dissimilarities between lactide and lactone, we can gain a deeper appreciation for the vast world of organic chemistry and the diverse compounds that shape it.
