Overview of Benzenoid and Non-Benzenoid Compounds
Aromatic compounds are organic compounds with a unique ring structure and exhibit specific aromatic properties. Benzenoid and non-benzenoid compounds are two categories of aromatic compounds that differ in their molecular structures and aromatic characteristics.
Benzenoid Compounds:
Definition and characteristics: Benzenoid compounds, also known as arenes, are aromatic compounds that contain a benzene ring or a fused ring system derived from benzene. These compounds exhibit high stability and are characterized by a planar ring structure with alternating double bonds.
Structure and bonding: Benzenoid compounds consist of a hexagonal ring of carbon atoms, with each carbon atom bonded to a hydrogen atom. The carbon-carbon bonds in the ring alternate between single and double bonds, creating a resonance structure.
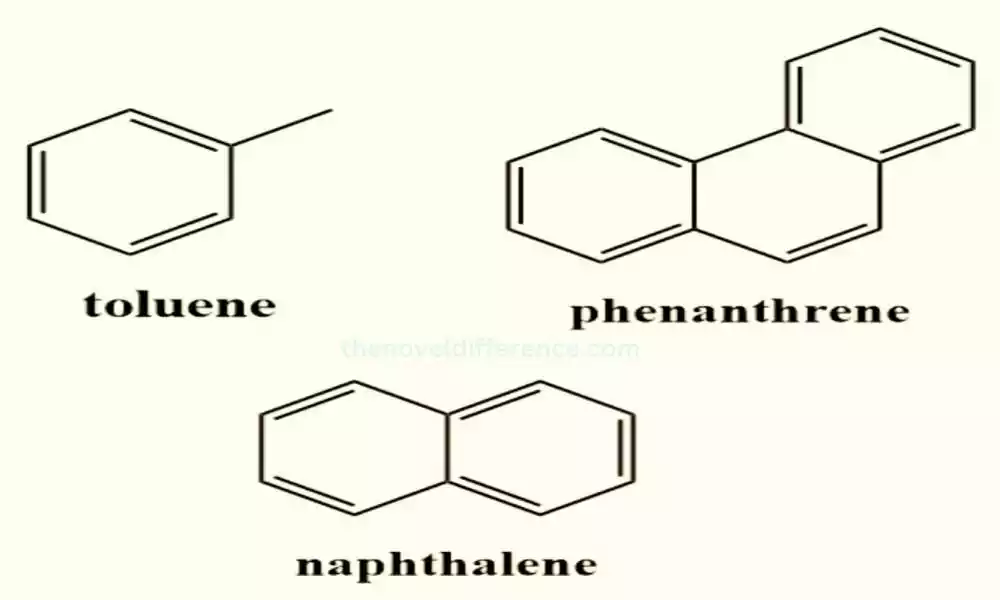
Examples: Benzene, toluene, and xylene are common benzenoid compounds. Chemical precursors and solvents play an essential part in manufacturing various polymers and chemicals; acting as solvents, fuels, or precursors during their respective production processes.
Aromaticity and Huckel’s rule: Benzenoid compounds satisfy the criteria of aromaticity, according to Huckel’s rule. This rule states that for a compound to be aromatic, it must have a planar, cyclic, and fully conjugated pi-electron system with 4n+2 π-electrons, where n is an integer.
Properties and reactivity: Benzenoid compounds are highly stable, have low reactivity, and exhibit unique properties such as resonance stabilization and delocalization of electrons. They undergo electrophilic aromatic substitution reactions and can participate in various organic reactions.
Non-Benzenoid Compounds:
Definition and characteristics: Non-benzenoid compounds are aromatic compounds that do not possess a benzene ring or its fused derivatives. They can have different ring structures, including cycloalkanes, cycloalkenes, and heterocycles, which exhibit aromaticity or antiaromaticity.
Structure and bonding: Non-benzenoid compounds have diverse forms, but they also contain cyclic arrangements of carbon atoms. The bonding within these compounds can include single, double, or triple bonds, depending on the specific structure.
Examples: Cyclohexane, cyclooctatetraene, and cyclopentadiene are examples of non-benzenoid compounds. Cyclohexane is a saturated cycloalkane, cyclooctatetraene is a polyene with alternating double bonds, and cyclopentadiene is a cyclic diene.
Aromaticity and antiaromaticity: Non-benzenoid compounds can exhibit aromaticity or antiaromaticity, depending on the number of pi-electrons in their ring system. Aromatic compounds follow the same criteria as benzenoid compounds (4n+2 π-electrons), while antiaromatic compounds have 4n π-electrons and are highly unstable.
Properties and reactivity: Non-benzenoid compounds can have diverse properties and reactivities based on their specific structures. Some non-benzenoid compounds can undergo aromatic substitution reactions, while others may exhibit different reactivity patterns due to the presence of multiple bonds or heteroatoms in the ring.
Understanding the differences between benzenoid and non-benzenoid compounds is crucial for studying aromaticity, designing new molecules, and predicting their properties and reactivity.
Definition of aromatic compounds
Aromatic compounds are organic molecules with specific molecular structures known as aromatic rings or systems, creating an aromatic fragrance. Aromaticity refers to the characteristic stability and unique chemical properties exhibited by these compounds.
The term “aromatic” was initially used to describe compounds with pleasant smells, but it was later discovered that aromaticity is a property related to the electronic structure of the molecule rather than its odor. Aromatic compounds can have a wide range of odors or even be odorless.
Aromatic compounds typically contain a cyclic arrangement of atoms, often carbon atoms, with alternating single and double bonds. Benzene rings are one of the best-known examples of aromatic rings; each contains six carbon atoms in a hexagonal configuration with alternate single and double bonds.
Aromatic systems can also include fused or substituted rings, and can even incorporate heteroatoms (atoms other than carbon) such as nitrogen, oxygen, or sulfur.
Aromatic compounds exhibit unique chemical and physical properties due to the presence of aromaticity. These properties include high stability, resonance delocalization of electrons, relatively low reactivity, and specific patterns of reactivity in certain types of chemical reactions.
Aromatic compounds are found across nature and have various applications across industries like pharmaceuticals, dyes, fragrances, polymers, and materials science.
Note that not all compounds with aromatic rings can be considered aromatic compounds. A compound must satisfy specific criteria, such as having a planar, cyclic structure and a certain number of pi-electrons in the conjugated system, to be considered aromatic.
These criteria are often defined by rules like Huckel’s rule, which states that for a compound to be aromatic, it must have a fully conjugated system with 4n+2 pi-electrons, where n is an integer.
Aromatic compounds are a class of organic compounds characterized by their stable and resonant cyclic structures, unique chemical properties, and often pleasant odors. They play a vital role in various industries and are a subject of significant interest in organic chemistry.
Importance of understanding the difference between benzenoid and non-benzenoid compounds
Understanding the difference between benzenoid and non-benzenoid compounds is important for several reasons:
Structure and Bonding: Benzenoid compounds possess an easily recognizable structure consisting of either an aromatic benzene ring, its fused derivatives, or multiple rings arranged randomly within it, while non-benzenoid compounds exhibit diverse structures with multiple rings arranged similarly. Recognizing these structural differences is crucial for understanding their physical properties, reactivity, and behavior in chemical reactions.
Aromaticity and Reactivity: Benzenoid compounds exhibit aromaticity, which imparts them with unique stability and reactivity. Non-benzenoid compounds can display aromaticity or antiaromaticity, leading to different reactivity patterns. Understanding these concepts helps in predicting the behavior of aromatic compounds and their involvement in various chemical reactions.
Synthetic Strategies: Distinguishing between benzenoid and non-benzenoid compounds is essential in designing synthetic routes for their preparation. Different strategies and reaction conditions may be required to synthesize specific types of aromatic compounds based on their structural classification. Knowledge of the differences aids in selecting appropriate synthetic methods.
Functional Group Compatibility: Benzenoid and non-benzenoid compounds often have different functional groups attached to their aromatic rings. Understanding the distinction allows for better comprehension of the compatibility and interactions between these functional groups and the aromatic system. This knowledge is valuable when designing and modifying molecules for specific applications.
Properties and Applications: Benzenoid and non-benzenoid compounds can exhibit different physical and chemical properties due to their structural dissimilarities. This dissimilarity impacts their applications in various fields such as pharmaceuticals, materials science, agrochemicals, and organic electronics. Understanding the differences assists in selecting the appropriate compound for a specific application based on its properties.
Medicinal Chemistry and Drug Discovery: Aromatic compounds are widely utilized in medicinal chemistry and drug discovery. Understanding the differences between benzenoid and non-benzenoid structures helps researchers design molecules with desired pharmacological properties. Optimize drug properties like bioavailability, potency, and selectivity with computational approaches to drug design.
Understanding benzenoid compounds versus non-benzenoid compounds is vital in order to gain insight into their structure, reactivity, properties, and applications.
This knowledge aids in synthetic planning, predicting chemical behavior, and optimizing the design of molecules for various purposes, including pharmaceuticals and materials science.
Benzenoid Compounds
Benzenoid compounds, also known as arenes, are a class of aromatic compounds that contain a benzene ring or fused ring systems derived from benzene. These compounds showcase unique properties and have many applications across various fields.
Here are some key features of benzenoid compounds:
Structure: Benzenoid compounds possess a distinct hexagonal carbon ring structure. Each carbon atom is bonded to a hydrogen atom, resulting in a fully saturated ring. The carbon-carbon bonds within the benzene ring alternate between single and double bonds, forming a resonance structure.
Aromaticity: Benzenoid compounds are aromatic, meaning they possess a high degree of stability due to aromaticity. Aromaticity arises from the delocalization of pi-electrons throughout the ring, resulting in a lower energy state. This delocalization of electrons gives benzenoid compounds their unique properties.
Examples:
Benzene (C6H6): This simple yet widely-recognized benzenoid compound stands out among others for being both accessible and familiar to most. It consists of a ring of six carbon atoms with alternating single and double bonds. Benzene is an opaque liquid with an aromatic sweetness used as the starting material to synthesize various chemicals and polymers.
Toluene (C7H8): Toluene is an analog of benzene with one hydrogen replaced by a methyl group (CH3). It is a volatile liquid with a distinct aromatic smell and is commonly used as a solvent and fuel additive.
Xylene (C₆H₄(CH₃)₂): Xylene is another derivative of benzene, with two methyl groups attached to the ring. It is a colorless liquid with a sweet odor and is used as a solvent in various industries, including paint and coating production.
Properties and Reactivity: Benzenoid compounds possess several characteristic properties and reactivity patterns:
Stability: Benzenoid compounds exhibit exceptional stability due to the resonance delocalization of pi-electrons. This stability makes them resistant to many chemical reactions.
Electrophilic Aromatic Substitution: Benzenoid compounds are particularly reactive towards electrophiles, undergoing electrophilic aromatic substitution reactions. These reactions involve the substitution of a hydrogen atom on the benzene ring with another group.
Resonance and Delocalization: The resonance structure of benzenoid compounds allows for the delocalization of pi-electrons throughout the ring. This delocalization contributes to their stability and unique properties.
Conjugation: Benzenoid compounds can undergo conjugation with other unsaturated systems, such as double bonds or other aromatic rings. This conjugation further enhances their stability and modifies their reactivity.
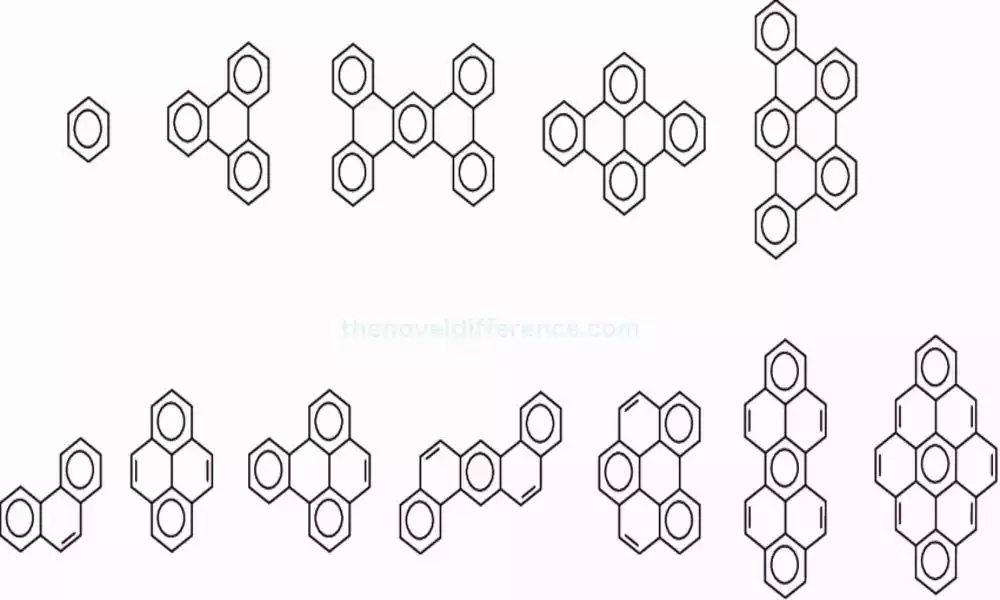
Benzenoid compounds play an integral part in organic chemistry and find applications across industries, such as pharmaceuticals, plastics, dyes, and fragrances. Understanding their structure, properties, and reactivity is essential for designing new molecules, predicting their behavior, and utilizing them effectively in various applications.
Definition and characteristics
Benzenoid compounds, also known as arenes, are a class of aromatic compounds that contain a benzene ring or fused ring systems derived from benzene. These compounds exhibit unique characteristics and properties that distinguish them from non-benzenoid compounds.
Here are the definitions and key characteristics of benzenoid compounds:
Definition: Benzenoid compounds are organic compounds that possess a cyclic structure consisting of a benzene ring or its fused derivatives. The benzene ring is a six-membered carbon ring with three alternating double bonds and three single bonds, resulting in a planar and conjugated system of π-electrons.
Characteristics:
Aromaticity: The most notable characteristic of benzenoid compounds is their aromaticity. Aromaticity refers to the special stability and reactivity conferred by a molecule’s conjugated π-electron system. Benzenoid compounds satisfy the criteria for aromaticity, which include having a planar, cyclic structure and a certain number of π-electrons.
Resonance Stabilization: Benzenoid compounds exhibit resonance stabilization due to the delocalization of π-electrons over the entire benzene ring. This resonance delocalization contributes to the exceptional stability of these compounds.
Unique Physical Properties: Benzenoid compounds often possess distinct physical properties. They are typically colorless or pale yellow liquids or solids with characteristic aromas. They have relatively high boiling points and are nonpolar or only slightly polar due to the uniform distribution of electron density in the aromatic ring.
Reactivity: Benzenoid compounds exhibit unique reactivity patterns. They are less reactive than non-aromatic compounds due to their exceptional stability resulting from aromaticity. Benzenoid compounds typically undergo electrophilic aromatic substitution reactions, where an electrophile replaces a hydrogen atom on the benzene ring. This reaction is a key characteristic of aromatic compounds.
Applications: Benzenoid compounds have extensive applications in various fields. Organic building blocks play an essential part in organic synthesis, serving as precursors for producing numerous chemicals, pharmaceuticals, polymers, and materials. Additionally, many benzenoid compounds have applications in the fragrance and flavor industries due to their characteristic aromas.
Understanding the definition and characteristics of benzenoid compounds is crucial for studying aromaticity, designing and synthesizing new molecules, predicting their reactivity, and utilizing them effectively in various applications. Their unique properties and stability make them highly valuable in the field of organic chemistry.
Non-Benzenoid Compounds
Non-benzenoid compounds are a class of aromatic compounds that do not possess a benzene ring or its fused derivatives. These compounds exhibit aromaticity or antiaromaticity based on their molecular structure.
Here are the definitions and key characteristics of non-benzenoid compounds:
Definition: Non-benzenoid compounds are organic compounds that exhibit aromaticity or antiaromaticity without containing a benzene ring or its fused derivatives. These compounds possess cyclic structures with different ring systems, including cycloalkanes, cycloalkenes, heterocycles, or other fused ring arrangements.
Characteristics:
Aromaticity or Antiaromaticity: Non-benzenoid compounds can exhibit aromaticity or antiaromaticity based on the number of π-electrons in their ring systems. Aromatic compounds follow the same criteria as benzenoid compounds, having a planar, cyclic structure and a certain number of π-electrons (4n + 2). On the other hand, antiaromatic compounds have a number of π-electrons (4n) that make them highly unstable.
Diverse Ring Structures: Non-benzenoid compounds can have various ring structures.
For example:
Cycloalkanes: Non-benzenoid compounds can consist of saturated cyclic hydrocarbons such as cyclohexane, cyclopentane, or cyclooctane. These compounds do not possess alternating double bonds and are not aromatic.
Cycloalkenes: Non-benzenoid compounds like cyclopentadiene or cyclooctatetraene have unsaturated cyclic structures with alternating single and double bonds, but they do not fulfill the criteria for aromaticity.
Heterocycles: Non-benzenoid compounds may contain heteroatoms (e.g., nitrogen, oxygen, sulfur) in their ring systems, creating diverse heterocyclic structures. These heterocycles can exhibit aromaticity or antiaromaticity depending on their π-electron count.
Properties and Reactivity: Non-benzenoid compounds can have varying physical and chemical properties based on their specific ring structures and aromatic or antiaromatic character. These compounds may exhibit different stability levels and reactivity patterns compared to benzenoid compounds. The reactivity of organic molecules may depend on various factors, including strain on their rings, the presence of double bonds, and the type and nature of substituents.
Applications: Non-benzenoid compounds find applications in various fields, including pharmaceuticals, materials science, and organic synthesis. Some non-benzenoid aromatic compounds, such as furan and pyrrole, have important roles in drug discovery and serve as building blocks for bioactive molecules.
Understanding the properties and reactivity of non-benzenoid compounds is crucial for studying their aromatic or antiaromatic behavior, predicting their physical and chemical properties, and utilizing them in organic synthesis and other applications.
These compounds expand the scope of aromatic chemistry beyond the traditional benzene ring and offer unique opportunities for molecular design and functionalization.
Definition and characteristics
Non-benzenoid compounds are a class of organic compounds that do not contain a benzene ring or its fused derivatives. These compounds possess diverse structures and exhibit varying degrees of aromaticity or antiaromaticity.
Here are the definitions and key characteristics of non-benzenoid compounds:
Definition: Non-benzenoid compounds are organic compounds that lack a benzene ring or its fused derivatives while still displaying aromaticity or antiaromaticity. These compounds may include various cyclic structures such as cycloalkanes, cycloalkenes, heterocycles, or fused ring systems.
Characteristics:
Aromaticity or Antiaromaticity: Non-benzenoid compounds can exhibit aromaticity or antiaromaticity, similar to benzenoid compounds, based on their cyclic structure and number of π-electrons. Aromatic compounds have a cyclic, planar structure with a specific number of π-electrons (4n + 2), resulting in increased stability. Antiaromatic compounds, on the other hand, possess a cyclic structure with a different number of π-electrons (4n), making them highly unstable.
Diverse Ring Structures: Non-benzenoid compounds display a wide range of ring structures.
Some examples include:
Cycloalkanes: Non-benzenoid compounds like cyclohexane or cyclooctane consist of saturated cyclic hydrocarbons without alternating double bonds. These compounds lack aromaticity but are important for their stability and use as structural components.
Cycloalkenes: Non-benzenoid compounds such as cyclopentadiene or cyclooctatetraene have unsaturated cyclic structures with alternating single and double bonds. While they do not fulfill the criteria for aromaticity, they may exhibit other interesting properties due to their conjugated π-electron system.
Heterocycles: Non-benzenoid compounds can contain heteroatoms (e.g., nitrogen, oxygen, sulfur) within their ring structures, leading to diverse heterocyclic compounds. These compounds can display aromatic or antiaromatic behavior based on their π-electron count and can have unique properties and reactivities.
Properties and Reactivity: Non-benzenoid compounds exhibit a range of physical and chemical properties depending on their specific structures. These compounds can have different stabilities, boiling points, and polarities compared to benzenoid compounds. Their reactivity can also vary, influenced by factors such as ring strain, the presence of double bonds, and the nature of substituents attached to the ring.
Applications: Non-benzenoid compounds find applications in various fields, including pharmaceuticals, materials science, and organic synthesis. Some heterocyclic compounds, like pyrrole and furan, have long been used as building blocks in drug discovery research and medicine chemistry.
Understanding the properties and reactivity of non-benzenoid compounds is crucial for studying their aromatic or antiaromatic behavior, predicting their physical and chemical properties, and exploring their applications in different fields.
These compounds provide a broader scope for aromatic chemistry beyond the traditional benzene ring, offering unique opportunities for molecular design and functionalization.
Differences Between Benzenoid and Non-Benzenoid Compounds
There are several key differences between benzenoid and non-benzenoid compounds, which are summarized below:
Molecular Structure and Bonding: Benzenoid compounds possess the distinctive structure of being composed of either an unfused benzene ring or fused rings derived from it. The benzene ring is a planar hexagonal ring with alternating single and double bonds.
Non-benzenoid compounds have diverse cyclic structures that may include cycloalkanes, cycloalkenes, heterocycles, or other fused ring arrangements. These compounds can have different numbers and types of bonds within the ring.
Aromaticity and Antiaromaticity: Benzenoid compounds satisfy the criteria for aromaticity, which include having a fully conjugated π-electron system, a planar, cyclic structure, and a specific number of π-electrons (4n + 2). They exhibit exceptional stability due to aromaticity.
Non-benzenoid compounds can exhibit aromaticity or antiaromaticity. Some non-benzenoid compounds, such as certain heterocycles, can possess aromatic character based on the number of π-electrons in their ring system. Others may be antiaromatic, possessing several π-electrons that fulfill the criteria for antiaromaticity (4n). Anti-aromatic compounds are highly unstable.
Reactivity and Stability: Benzenoid compounds are generally highly stable due to aromaticity, making them less reactive compared to non-aromatic compounds. They undergo electrophilic aromatic substitution reactions and exhibit unique patterns of reactivity.
Non-benzenoid compounds can have varied stability and reactivity based on their specific structures. Factors such as ring strain, the presence of double bonds, and heteroatoms can influence their stability and reactivity.
Physical Properties: Benzenoid compounds often have distinct physical properties. They are typically colorless or pale yellow liquids or solids with characteristic aromas. They have relatively high boiling points and are nonpolar or only slightly polar due to the uniform distribution of electron density in the aromatic ring.
Non-benzenoid compounds can have diverse physical properties depending on their specific structures. Cycloalkanes have different physical properties compared to benzenoid compounds due to the absence of π-electrons and the presence of saturated carbon-carbon bonds.
Applications: Benzenoid compounds find widespread applications in various fields, including pharmaceuticals, materials science, fragrances, and polymers. Their stability, unique reactivity, and aromatic character make them valuable building blocks.
Non-benzenoid compounds also have important applications in various industries. For instance, certain heterocyclic compounds are widely used in drug discovery and serve as building blocks for bioactive molecules.
Understanding these key differences between benzenoid and non-benzenoid compounds is essential for studying aromaticity, designing new molecules, predicting their properties and reactivity, and utilizing them effectively in different applications.
What are the similarities between Benzenoid and Non-Benzenoid?
While benzenoid and non-benzenoid compounds differ in terms of their molecular structures and aromatic characteristics, they do share some similarities.
Here are a few similarities between benzenoid and non-benzenoid compounds:
Aromatization: Aromatic compounds from both benzenoid and non-benzenoid families may exhibit aromaticity. Aromatic compounds, regardless of their specific structures, possess a conjugated π-electron system and satisfy certain criteria, such as a planar, cyclic structure and a specific number of π-electrons (4n + 2 for aromatic compounds, 4n for antiaromatic compounds). Aromaticity imparts stability and unique chemical properties to these compounds.
Conjugated P-Electron System: Both benzenoid and non-benzenoid compounds possess an interconvertible conjugated p-electron system. The presence of alternating single and double bonds or other forms of conjugation allows for the delocalization of π-electrons, contributing to the stability and reactivity of these compounds.
Resonance: Both benzenoid and non-benzenoid compounds can exhibit resonance. Resonance refers to the delocalization of electrons within a molecule, resulting in multiple equivalent resonance structures. The resonance stabilization contributes to the stability and unique reactivity of these compounds.
Chemical Reactivity: Both types of compounds can participate in various organic reactions. Benzenoid compounds undergo electrophilic aromatic substitution reactions, where an electrophile substitutes a hydrogen atom on the benzene ring. Non-benzenoid compounds can also undergo different types of reactions, depending on their specific structures, such as addition reactions or ring-opening reactions.
Applications: Both benzenoid and non-benzenoid compounds find applications in various fields, including pharmaceuticals, materials science, and organic synthesis. These compounds serve as fundamental building blocks in the production of various chemicals, polymers, and bioactive compounds.
It’s important to note that while benzenoid and non-benzenoid compounds share some similarities, their differences in molecular structure and aromatic characteristics ultimately lead to distinct properties and reactivities.
Understanding both their similarities and differences is crucial for comprehending aromaticity and utilizing these compounds effectively in various applications.
Comparison – Benzenoid vs Non-Benzenoid in Tabular Form
Here’s a comparison between benzenoid and non-benzenoid compounds in tabular form:
| aspect | Benzenoid Compounds | Non-Benzenoid Compounds |
|---|---|---|
| Definition | Compounds with a benzene ring or fused derivatives | Compounds without a benzene ring or its derivatives |
| Structure | Consists of a benzene ring or fused ring system derived from benzene | Diverse cyclic structures, including cycloalkanes, cycloalkenes, heterocycles, or other fused ring systems |
| Aromaticity | Exhibits aromaticity, satisfying the criteria for aromatic compounds (4n + 2 π-electrons) | Can exhibit aromaticity or antiaromaticity based on the number of π-electrons in the ring system |
| Reactivity | Undergo electrophilic aromatic substitution reactions | Varied reactivity based on specific structures |
| Stability | Highly stable due to aromaticity | Stability can vary based on specific structures |
| Physical Properties | Colorless or pale yellow, characteristic aroma, relatively high boiling points | Diverse physical properties based on specific structures |
| Applications | Widely used in pharmaceuticals, fragrances, materials science, etc. | Applications in various industries, including pharmaceuticals and organic synthesis, depending on specific structures |
This table gives a quick snapshot of the key distinctions between benzenoid and non-benzenoid compounds, emphasizing their unique structural, aromaticity, reactivity, stability physical properties, and application characteristics.
Conclusion
Benzenoid and non-benzenoid compounds are two categories of aromatic compounds with distinct characteristics and properties. Benzenoid compounds consist of a benzene ring or its fused derivatives and exhibit aromaticity, high stability, and unique reactivity.
Examples of benzenoid compounds include benzene, toluene, and xylene. They find widespread applications in various industries, including pharmaceuticals, fragrances, and materials science.
