Explore the key differences between isosmotic hyperosmotic and hypoosmotic solutions. This comprehensive article provides in-depth insights into these terms, their implications, and their relevance in various biological and physiological contexts.
Introduction
Isosmotic hyperosmotic and hypoosmotic solutions are terminologies commonly used in biology and physiology to describe the osmolarity or concentration of solutes in different solutions. Understanding the differences between these solutions is crucial to grasping their significance in maintaining proper cell function, fluid balance, and physiological homeostasis.
We will delve into the contrasts between isosmotic hyperosmotic and hypoosmotic solutions. We will explore their definitions, underlying principles, and their implications in biological systems. So, let’s dive right in!
What are Isosmotic Solutions?
Isosmotic solutions refer to solutions that have the same osmolarity, or concentration of solute particles, as another solution or system with which they are being compared. The concentration of solute particles is equal on both sides of a semipermeable membrane, resulting in no net movement of water across the membrane.
Isosmotic solutions are important for maintaining cellular homeostasis and preventing water imbalance. They play a significant part in different natural forms, such as keeping up the right working of cells and tissues. Cells can maintain their normal shape and volume, and there is no net movement of water into or out of the cells.
The body fluids, such as blood and extracellular fluid, are typically isosmotic. This allows for the proper functioning of cells, as the concentration of solutes inside the cells is balanced with the surrounding environment. Isosmotic solutions are also used in medical and research settings, such as in intravenous fluids, where maintaining the osmotic balance is critical for patient health.

Isosmotic solutions have the same osmolarity as the reference solution or system, resulting in a balanced concentration of solutes on both sides of a membrane and no net movement of water. They are important for maintaining cellular homeostasis and are found in various biological and medical contexts.
Characteristics of an isosmotic solution
Characteristics of an isosmotic solution include:
1. Equal Osmolarity: An isosmotic solution has the same osmolarity as the reference solution or system with which it is being compared. This means that the concentration of solute particles in the isosmotic solution is balanced and equal to the concentration on the other side of a semipermeable membrane.
2. No Net Movement of Water: There is no net movement of water across a semipermeable membrane. This is because the concentrations of solutes on both sides of the membrane are equal, resulting in no osmotic pressure gradient that would drive water movement.
3. Maintenance of Cellular Shape and Volume: Isosmotic solutions are crucial for maintaining the proper shape and volume of cells. Since there is no net movement of water, cells in an isosmotic solution can maintain their normal morphology and structural integrity.
4. Cellular Homeostasis: Isosmotic solutions are essential for cellular homeostasis. They help regulate the balance of water and solute concentrations within cells, ensuring that the internal environment remains stable and functional. Isosmotic conditions allow cells to carry out their physiological processes efficiently.
5. Physiological Compatibility: Isosmotic solutions are compatible with living organisms and cells. They closely resemble the osmolarity of body fluids, such as blood and extracellular fluid, enabling them to support normal cellular functions without causing damage or disruption.
6. Preventing Water Imbalance: Isosmotic solutions play a role in preventing water imbalance in biological systems. They help maintain the balance between intracellular and extracellular fluid compartments, ensuring that water is distributed appropriately and preventing excessive water loss or gain.
7. Importance in Medical Applications: Isosmotic solutions have practical applications in medical settings. Intravenous fluids used in medical treatments are often isosmotic to maintain the osmotic balance in the body and prevent complications associated with fluid imbalances.
Isosmotic solutions have characteristics such as equal osmolarity, no net movement of water, maintenance of cellular shape and volume, support for cellular homeostasis, physiological compatibility, prevention of water imbalance, and relevance in medical applications. These characteristics are essential for maintaining the proper functioning of cells and ensuring overall biological balance.
Examples of isosmotic solutions in living organisms
There are several examples of isosmotic solutions in living organisms.
Here are a few:
1. Intracellular Fluid (ICF): The cytoplasm, or the fluid inside the cells, is generally considered isosmotic. It has an osmolarity that is balanced with the surrounding extracellular environment, allowing for proper cellular function and maintaining cell shape and volume.
2. Extracellular Fluid (ECF): The fluid that surrounds cells, including interstitial fluid and plasma, is typically isosmotic. It has a similar osmolarity to the cytoplasm, ensuring a balanced exchange of nutrients, waste products, and signaling molecules between cells and their external environment.
3. Blood Plasma: Blood plasma, the liquid component of blood, is an example of an isosmotic solution. It keeps up an osmolarity that’s consistent with the encompassing cells and tissues, permitting for proficient transport of oxygen, supplements, hormones, and other substances all through the body.
4. Lymph Fluid: Lymph is the fluid that circulates through the lymphatic system. Similar to the extracellular fluid, and plays a crucial role in immune response, waste removal, and maintaining fluid balance in the body.
5. Amniotic Fluid: Amniotic fluid, which surrounds and protects the developing fetus during pregnancy, is also isosmotic. It maintains a balanced osmolarity to support the growth and development of the fetus and provide a stable environment for its cells.
It is imperative to note that the osmolarity of these liquids may shift somewhat depending on particular physiological conditions or outside components. Their overall osmolarity remains relatively stable and is adjusted to maintain cellular homeostasis and support proper biological functions.
What is Hyperosmotic Solutions?
Hyperosmotic solutions refer to solutions or systems that have a higher osmolarity, or concentration of solute particles, compared to another solution or system with which they are being compared.
The concentration of solute particles is more prominent on one side of a semipermeable layer, causing water to move from a range of lower solute concentration (lower osmolarity) to a range of higher solute concentration (higher osmolarity) in an endeavor to equalize the concentrations.
Key characteristics of hyperosmotic solutions include:
1. Higher Solute Concentration: Hyperosmotic solutions have a higher concentration of solute particles compared to the reference solution or system. The greater number of solute particles results in an increased osmolarity.
2. Net Movement of Water Outward: Due to the osmotic weight slope, water moves from ranges of lower solute concentration (lower osmolarity) to regions of higher solute concentration (higher osmolarity). This leads to a net movement of water out of cells or across a membrane into the hyperosmotic solution.
3. Cell Shrinkage or Dehydration: When cells are exposed to a hyperosmotic solution, water moves out of the cells, causing them to shrink or dehydrate. The higher solute concentration exterior of the cells makes an osmotic slope that draws water out of the cells, driving a diminish in cell volume.
4. Effects on Biological Systems: Hyperosmotic solutions can have various effects on biological systems. Excessive exposure to hyperosmotic conditions can disrupt cellular functions and damage cell structures. Hyperosmotic environments, such as highly salty or hypertonic habitats, can pose challenges to water balance and may require osmoregulatory adaptations to prevent dehydration.
5. Clinical and Experimental Applications: Hyperosmotic solutions find applications in various clinical and experimental settings. Hypertonic saline solutions are used in medical interventions to draw fluid out of tissues and reduce swelling. Hyperosmotic solutions are also utilized in laboratory experiments to manipulate osmotic conditions and study cellular responses to changes in osmolarity.
Hyperosmotic solutions have a higher concentration of solute particles compared to the reference solution or system. They cause a net movement of water outwards, resulting in cell shrinkage or dehydration. Hyperosmotic solutions can have important implications for biological systems, requiring adaptations to maintain water balance and find applications in clinical and experimental contexts.

Characteristics of a hyperosmotic solution
Characteristics of a hyperosmotic solution include:
1. Higher Osmolarity: A hyperosmotic solution has a higher osmolarity compared to the reference solution or system with which it is being compared. The osmolarity is decided by the concentration of solute particles display within the arrangement. The concentration of solute particles is greater, resulting in an increased osmolarity.
2. Net Movement of Water: Due to the osmotic weight slope, water moves from zones of lower solute concentration (lower osmolarity) to ranges of higher solute concentration (higher osmolarity). Water tends to move out of the cells or across a membrane into the hyperosmotic solution to equalize the solute concentrations.
3. Cell Shrinkage or Dehydration: When cells are exposed to a hyperosmotic solution, water moves out of the cells. This causes the cells to shrink or dehydrate as the water exits the cell to dilute the higher concentration of solutes in the surrounding solution. The higher solute concentration exterior of the cells makes an osmotic slope that draws water out of the cells, coming about in a diminish in cell volume.
4. Osmotic Pressure: Hyperosmotic solutions exert a higher osmotic pressure compared to the reference solution or system. Osmotic pressure is the force generated by the movement of water across a semipermeable membrane due to differences in solute concentrations. The higher the osmolarity of an arrangement, the more noteworthy the osmotic weight it applies.
5. Effects on Biological Systems: Exposure to hyperosmotic solutions can have various effects on biological systems. Prolonged exposure to hyperosmotic conditions can disrupt cellular functions, affect enzyme activity, and potentially damage cell structures. Being in a hyperosmotic environment, such as highly salty or hypertonic habitats, can pose challenges to water balance and require osmoregulatory adaptations to prevent dehydration.
6. Clinical and Experimental Applications: Hyperosmotic solutions have practical applications in clinical and experimental settings. Hypertonic saline solutions are used medically to draw fluid out of tissues and reduce edema (swelling). Hyperosmotic solutions are also employed in laboratory experiments to manipulate osmotic conditions and study cellular responses to changes in osmolarity.
Hyperosmotic solutions have higher osmolarity, leading to a net movement of water out of cells, resulting in cell shrinkage or dehydration. They exert higher osmotic pressure and can have various effects on biological systems. Hyperosmotic solutions find applications in medical interventions and experimental research involving osmotic manipulation.
Examples of hyperosmotic solutions in biological systems
Examples of hyperosmotic solutions in biological systems include:
1. Hypertonic Saline Solution: Hypertonic saline solutions, which have a higher concentration of solute particles compared to physiological fluids, are used in medical settings. They can be administered intravenously to draw fluid out of tissues, reduce edema, and increase blood pressure. Hypertonic saline is additionally utilized in certain medical procedures, such as within the treatment of hyponatremia (moo sodium levels) or for revival purposes.
2. Urine: Urine is a hyperosmotic solution produced by the kidneys. The concentration of solutes in pee is regularly higher than within the plasma, coming about in a hyperosmotic state. The osmolarity of urine is regulated by the kidneys to control water balance and eliminate waste products from the body.
3. Saltwater (Hypertonic) Environments: Seawater is hyperosmotic compared to the internal fluids of marine organisms. The high concentration of dissolved salts, such as sodium chloride, in seawater, creates a hyperosmotic environment. Marine organisms, such as fish and marine invertebrates, have adaptations to osmoregulate and maintain their internal osmolarity in these hypertonic environments.
4. Concentrated Sugar Solutions: Concentrated sugar solutions, such as high-sugar syrups or concentrated fruit juices, can act as hyperosmotic solutions. These solutions have a higher concentration of sugar molecules compared to surrounding fluids. When fruits are preserved in sugar syrup, the hyperosmotic solution helps to inhibit bacterial growth and preserve the fruit’s freshness.
5. Hypertonic Solutions for Cell Preservation: Hypertonic solutions are used to dehydrate cells, reducing their metabolic activity and extending their viability. These solutions contain higher concentrations of solutes than the intracellular fluid, causing water to move out of the cells and promoting a state of suspended animation for long-term storage.
It is important to note that the examples provided can vary in their specific osmolarities and compositions depending on the context and the organisms involved. These examples demonstrate the presence and significance of hyperosmotic solutions in biological systems and their diverse applications.
What are Hypoosmotic Solutions?
Hypoosmotic solutions refer to solutions or systems that have a lower osmolarity, or concentration of solute particles, compared to another solution or system with which they are being compared. The concentration of solute particles is lower on one side of a semipermeable layer, causing water to move from a region of higher solute concentration (higher osmolarity) to a region of lower solute concentration (lower osmolarity) in an endeavor to equalize the concentrations.
Key characteristics of hypoosmotic solutions include:
1. Lower Osmolarity: A hypoosmotic solution has a lower osmolarity compared to the reference solution or system with which it is being compared. The osmolarity is decided by the concentration of solute particles display within the arrangement. The concentration of solute particles is lower, resulting in a decreased osmolarity.
2. Net Movement of Water Inward: Due to the osmotic weight slope, water moves from zones of higher solute concentration (higher osmolarity) to ranges of lower solute concentration (lower osmolarity). Water tends to move into the cells or across a membrane into the hypoosmotic solution to equalize the solute concentrations.
3. Cell Swelling or Hydration: When cells are exposed to a hypoosmotic solution, water moves into the cells. This causes the cells to swell or become hydrated as water enters the cell to dilute the lower concentration of solutes in the surrounding solution. The lower solute concentration outside the cells creates an osmotic gradient that draws water into the cells, increasing cell volume.
4. Osmotic Pressure: Hypoosmotic solutions exert a lower osmotic pressure compared to the reference solution or system. Osmotic pressure is the force generated by the movement of water across a semipermeable membrane due to differences in solute concentrations. The lower the osmolarity of an arrangement, the lower the osmotic weight it applies.
5. Effects on Biological Systems: Exposure to hypoosmotic solutions can have various effects on biological systems. Prolonged exposure to hypoosmotic conditions can cause cells to swell, potentially leading to cell rupture or bursting. Hypoosmotic environments, such as freshwater habitats, can pose challenges to water balance and may require osmoregulatory adaptations to prevent overhydration.
6. Clinical and Experimental Applications: Hypoosmotic solutions have practical applications in clinical and experimental settings. Hypotonic solutions, which are hypoosmotic, may be used in medical interventions to rehydrate patients or restore fluid balance. Hypoosmotic solutions are also employed in laboratory experiments to manipulate osmotic conditions and study cellular responses to changes in osmolarity.
Hypoosmotic solutions have lower osmolarity, leading to a net movement of water into cells, resulting in cell swelling or hydration. They exert lower osmotic pressure and can have various effects on biological systems. Hypoosmotic solutions find applications in medical interventions and experimental research involving osmotic manipulation.
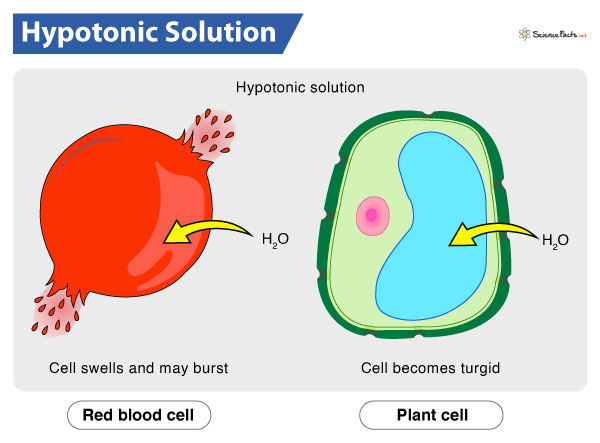
Characteristics of a hypoosmotic solution
Characteristics of a hypoosmotic solution include:
1. Lower Osmolarity: A hypoosmotic solution has a lower osmolarity compared to the reference solution or system it is being compared to. The concentration of solute particles in the hypoosmotic solution is lower, resulting in a decreased osmolarity.
2. Net Movement of Water Inward: Due to the osmotic pressure gradient, water moves from areas of higher solute concentration (higher osmolarity) to areas of lower solute concentration (lower osmolarity). Water tends to move into the cells or across a membrane into the hypoosmotic solution in an attempt to equalize the solute concentrations.
3. Cell Swelling or Hydration: When cells are exposed to a hypoosmotic solution, water moves into the cells, causing them to swell or become hydrated. The lower solute concentration outside the cells creates an osmotic gradient that draws water into the cells, leading to an increase in cell volume.
4. Osmotic Pressure: Hypoosmotic solutions exert a lower osmotic pressure compared to the reference solution or system. Osmotic pressure is the force exerted by the movement of water across a semipermeable membrane due to differences in solute concentrations. The lower the osmolarity of an arrangement, the lower the osmotic weight it applies.
5. Effects on Biological Systems: Exposure to hypoosmotic solutions can have various effects on biological systems. Prolonged exposure to hypoosmotic conditions can cause cells to swell, potentially leading to cell rupture or bursting. Hypoosmotic environments, such as freshwater habitats, can pose challenges to water balance and may require osmoregulatory adaptations to prevent overhydration.
6. Clinical and Experimental Applications: Hypoosmotic solutions have practical applications in clinical and experimental settings. Hypotonic solutions, which are hypoosmotic, may be used in medical interventions to rehydrate patients or restore fluid balance. Hypoosmotic solutions are also employed in laboratory experiments to manipulate osmotic conditions and study cellular responses to changes in osmolarity.
It is important to note that the specific osmolarity and composition of hypoosmotic solutions may vary depending on the context and the organisms involved. These characteristics highlight the presence and significance of hypoosmotic solutions in biological systems and their diverse applications.
Examples of hypoosmotic solutions in living organisms
Examples of hypoosmotic solutions in living organisms include:
1. Intracellular Fluid: The cytoplasm, which is the fluid inside cells, is considered a hypoosmotic solution compared to extracellular fluids. The intracellular liquid features a lower osmolarity due to the nearness of different solutes and macromolecules interior the cells.
2. Freshwater Environments: Freshwater bodies, such as rivers, lakes, and ponds, contain hypoosmotic solutions compared to the internal fluids of most organisms. Freshwater has a lower concentration of dissolved salts and other solutes compared to the fluids inside the cells of aquatic organisms. This creates a hypoosmotic environment, and organisms living in freshwater habitats need to regulate their water balance to prevent excessive water influx into their cells.
3. Plant Cells: Plant cells are surrounded by a cell wall and have a central vacuole, which can contain a hypoosmotic solution. The vacuole plays a crucial role in maintaining cell turgidity and providing structural support to the plant. The solute concentration in the vacuole is lower compared to the surrounding cytoplasm, resulting in a hypoosmotic condition.
4. Red Blood Cells (in Hypotonic Solution): Red blood cells, or erythrocytes, can experience a hypoosmotic environment when placed in a hypotonic solution. A hypotonic solution has a lower concentration of solutes compared to the cytoplasm of red blood cells. When red blood cells are exposed to a hypotonic solution, water moves into the cells, causing them to swell or potentially burst.
5. Some Marine Invertebrates: Some marine invertebrates, such as certain sponges and cnidarians, can maintain hypoosmotic internal fluids despite living in a hypertonic seawater environment. These organisms have adaptations to regulate their internal osmolarity and prevent excessive water loss to the surrounding hyperosmotic seawater.
These examples demonstrate the presence of hypoosmotic solutions in living organisms and highlight the need for osmoregulatory mechanisms to maintain water balance and prevent cell damage.
Comparison Chart
Here is a comparison chart highlighting the differences between isosmotic hyperosmotic and hypoosmotic solutions:
| Characteristic | Isosmotic Solution | Hyperosmotic Solution | Hypoosmotic Solution |
|---|---|---|---|
| Osmolarity | Same as a reference solution | Higher than reference solution | Lower than reference solution |
| Water Movement | No net movement of water | Water moves out of the solution | Water moves into the solution |
| Cell Response | No change in cell volume | Cell shrinkage or dehydration | Cell swelling or hydration |
| Osmotic Pressure | Equal on both sides | Higher on the hyperosmotic side | Lower on the hypoosmotic side |
| Effects on Biological Systems | Minimal impact | Cell shrinkage, potential damage | Cell swelling, potential rupture |
| Examples | Intracellular fluid | Hypertonic saline solution | Freshwater environments |
Importance of osmolarity in biological systems
Osmolarity plays a crucial role in maintaining the proper functioning of biological systems.
Here are some key reasons why osmolarity is important:
1. Cellular Homeostasis: Osmolarity is essential for maintaining the balance of water and solutes within cells. Cells have specific internal osmolarities that they must maintain to function optimally. Deviations from the appropriate osmotic balance can lead to cellular dysfunction and even cell death. Osmolarity ensures that water and solute concentrations are regulated within cells, allowing them to carry out their physiological processes.
2. Cell Volume Regulation: Osmolarity is directly linked to cell volume regulation. When cells are exposed to solutions with different osmolarities, water moves across the cell membrane to equalize the solute concentrations on both sides. This movement of water influences cell volume. Cells have mechanisms to regulate their volume and prevent excessive swelling or shrinkage caused by changes in osmolarity. Proper cell volume is crucial for maintaining cellular integrity, optimal enzymatic activity, and normal cellular functions.
3. Fluid Balance: Osmolarity plays a vital role in maintaining fluid balance within organisms. Different tissues and organs have specific osmolarities to function properly. Osmolarity gradients between compartments, such as the intracellular and extracellular spaces, help regulate the movement of water and solutes across membranes, ensuring proper fluid distribution and balance within the body. Osmotic pressure, determined by osmolarity, helps regulate fluid movement between compartments.
4. Osmoregulation in Organisms: Many organisms, especially those living in environments with varying osmolarities, have developed osmoregulatory mechanisms to maintain their internal osmolarity within a narrow range. These mechanisms allow them to adapt to different osmotic conditions and prevent detrimental effects caused by excessive water loss or gain. Osmoregulation is fundamental for the survival and legitimate working of life forms in different environments, counting freshwater, marine, and earthbound situations.
5. Transport Processes: Osmolarity influences various transport processes in biological systems. The osmotic gradient across cell membranes drives the movement of water and solutes through processes like osmosis and facilitated diffusion. Osmolarity angles too play a part in the assimilation of supplements from the stomach-related framework and the reabsorption of water within the kidneys.
Osmolarity is critical for maintaining cellular and organismal homeostasis, regulating cell volume, balancing fluid distribution, supporting osmoregulation, and facilitating essential transport processes in biological systems. It ensures that water and solute concentrations are regulated appropriately, allowing cells and organisms to function optimally in their respective environments.
Conclusion
Understanding the differences between isosmotic hyperosmotic and hypoosmotic solutions is crucial in comprehending the physiological processes and fluid dynamics within living organisms. These terms help describe the osmolarity or concentration of solutes in various solutions and their impacts on cellular function, fluid balance, and homeostasis.
Isosmotic solutions maintain equilibrium, while hyperosmotic solutions draw water from less concentrated solutions and hypoosmotic solutions draw water into more dilute solutions. Each type of solution has distinct cellular consequences and implications in biological and medical contexts.
By grasping the nuances of these terms, researchers, healthcare professionals, and students can gain a deeper understanding of the complexities of osmolarity and its role in maintaining proper cellular function and fluid balance.
