Hydrometallurgy and Pyrometallurgy. Both play crucial roles in extracting valuable metals from ores and have their unique advantages and applications. Understanding the contrast between hydrometallurgy and pyrometallurgy is basic for experts, analysts, and anybody inquisitive about the captivating world of metallurgy. We will delve deep into these processes, explore their distinctions, and shed light on their characteristics and applications.
Definition of Hydrometallurgy and Pyrometallurgy
Hydrometallurgy: Hydrometallurgy may be a department of extractive metallurgy that includes the utilization of fluid arrangements (ordinarily acids or bases) to extricate metals from their metals. It is a process that utilizes chemical reactions in a liquid medium to dissolve and recover metals. Hydrometallurgical processes typically involve ore preparation, leaching (where the metal is extracted into solution), solution purification, and metal recovery through precipitation or other techniques.
Pyrometallurgy: Pyrometallurgy may be a department of extractive metallurgy that utilizes tall temperatures to extricate metals from their minerals and concentrates. It includes utilizing warm and different chemical responses in a strong state to partition and extricate metals. Pyrometallurgical processes typically involve ore preparation, roasting (heating the ore to drive off impurities or convert it into a more suitable form), smelting (melting the ore to separate the metal), and refining (purifying the metal through further processes).
Hydrometallurgy includes the utilization of watery arrangements to extricate metals, whereas pyrometallurgy includes high-temperature forms to extricate metals from minerals.
Importance of metallurgical processes
Metallurgical processes play a crucial role in various industries and have significant importance in society.
Here are some key reasons why metallurgical processes are important:
1. Metal Production: Metallurgical processes are essential for the production of metals. They empower the extraction, refining, and change of metallic minerals into usable metals, such as press, copper, aluminum, gold, silver, and numerous others. These metals serve as crude materials for different businesses, counting development, car, aviation, hardware, and fabricating.
2. Economic Impact: The metallurgical industry contributes significantly to the global economy. Metal production and processing generate employment opportunities, foster economic growth, and drive industrial development in both developed and developing countries. The sale and export of metals and metal products contribute to national revenue and trade balances.
3. Infrastructure and Construction: Metallurgical processes provide the materials needed for infrastructure development and construction projects. Metals like steel and aluminum are broadly utilized in buildings, bridges, streets, railroads, pipelines, and other basic structures due to their quality, strength, and flexibility. These materials form the backbone of modern infrastructure.
4. Manufacturing and Engineering: Metallurgy is fundamental to manufacturing and engineering industries. Metals are essential for the production of machinery, equipment, tools, and components used in various sectors. Metallurgical processes enable the fabrication, shaping, and joining of metals, allowing the creation of intricate and customized parts for diverse applications.
5. Technological Advancements: Metallurgical processes drive technological advancements and innovation. They encourage the improvement of modern combinations and materials with improved properties, such as made strides in quality, erosion resistance, warm resistance, electrical conductivity, and more. These advancements enable the creation of advanced technologies, including renewable energy systems, electronic devices, vehicles, and medical equipment.
6. Recycling and Sustainability: Metallurgical processes play a vital role in metal recycling and sustainability efforts. They allow the recovery and reuse of valuable metals from discarded products and industrial waste, reducing the reliance on primary metal extraction. Recycling metals helps conserve natural resources, reduce energy consumption, minimize environmental impact, and promote circular economy principles.
7. Research and Development: Metallurgical processes continually undergo research and development to enhance efficiency, reduce costs, and improve environmental sustainability. Progressing progressions in metallurgy contribute to preparation optimization, the improvement of modern extraction strategies, and the disclosure of elective materials, clearing the way for a more feasible and proficient utilization of metals.
Metallurgical processes are essential for metal production, economic growth, infrastructure development, manufacturing, technological advancements, recycling, and sustainability. They have a noteworthy effect on different businesses and contribute to the change in living benchmarks and the progression of society.
What is Hydrometallurgy?
Hydrometallurgy may be a department of extractive metallurgy that includes the utilization of watery arrangements (as a rule acids or bases) to extricate metals from their minerals or concentrates. It is a process that utilizes chemical reactions in a liquid medium to dissolve and recover metals.
The ore or concentrate is typically subjected to various steps. The ore is prepared through crushing, grinding, and sometimes pre-treatment to make it more amenable to leaching. The next step is leaching, where the finely ground ore is exposed to a suitable solvent or leaching agent. The leaching agent selectively reacts with the metal of interest, dissolving it into a solution while leaving behind the impurities.
After leaching, the solution containing the dissolved metal is subjected to purification steps to remove impurities, such as solid particles, unwanted metals, or contaminants. This purification can involve filtration, solvent extraction, ion exchange, precipitation, or electrochemical methods.
Once the arrangement is decontaminated, the ultimate step is metal recuperation, where the metal is isolated from the arrangement and changed over into a usable frame. This can be achieved through techniques like precipitation, electro-winning (electroplating the metal onto a cathode), or other specific methods depending on the nature of the metal and the desired end product.
Hydrometallurgy offers several advantages compared to other extraction methods. It can be used to recover metals from low-grade ores or ores with complex compositions that may not be amenable to traditional pyrometallurgical processes. It is often a more environmentally friendly option as it generally requires lower energy consumption, generates fewer emissions, and can utilize recycled water. Hydrometallurgical processes are also more flexible and can be adjusted to handle different ore types and recover a wide range of metals.
Hydrometallurgy finds applications in different businesses, including mining, mineral handling, chemical handling, natural remediation, and reusing. It is commonly utilized for the extraction of metals like copper, gold, silver, nickel, cobalt, zinc, uranium, and uncommon soil components.

Hydrometallurgy could be a vital preparation within the extraction and recuperation of metals, giving an effective and ecologically neighborly approach to getting profitable metals from minerals and concentrates.
Process description
The process of hydrometallurgy typically involves the following steps:
1. Ore Preparation: The ore or concentrate is prepared to facilitate efficient leaching. This may involve crushing, grinding, and sometimes pre-treatment steps such as roasting, calcination, or bioleaching. Ore preparation aims to expose the maximum surface area of the ore particles for better contact with the leaching solution.
2. Leaching: The prepared ore is subjected to leaching, where it is contacted with a suitable solvent or leaching agent. Commonly used leaching agents are aqueous solutions of acids, bases, or complexing agents. The leaching agent selectively reacts with the metal of interest, dissolving it into a solution while leaving behind the impurities.
3. Solution Purification: The leach solution obtained from the previous step may contain impurities, such as solid particles, unwanted metals, or contaminants. Purification steps are employed to remove these impurities and obtain a solution enriched with the desired metal. Purification techniques may include filtration, solvent extraction, ion exchange, precipitation, or electrochemical methods.
4. Metal Recovery: Once the solution is purified, the final step is the recovery of the metal from the solution. The specific method used for metal recovery depends on the nature of the metal and the desired end product. Common techniques for metal recovery in hydrometallurgy include precipitation, electro-winning, or other specialized methods. Precipitation involves the addition of a chemical reagent that causes the metal to form a solid compound, which can then be separated by filtration or other separation techniques. Electro-winning involves passing an electric current through the solution, causing the metal ions to plate onto a cathode, resulting in the deposition of the metal in a pure form.
5. Post-Treatment: The recovered metal may require further purification or processing to meet the desired quality or specifications. Post-treatment steps may include refining, smelting, alloying, or other processes to enhance the purity and properties of the metal.
Throughout the entire process, careful monitoring and control of various parameters such as temperature, pH, agitation, and residence time are essential to optimize extraction efficiency and maintain the desired reaction conditions. The process may also involve recycling or treatment of spent leach solutions to minimize waste and environmental impact.
It’s imperative to note that the particular subtle elements and varieties of the method can shift depending on the sort of mineral, the target metal, and the specified conclusion item. Different metals and ores may require specific leaching agents, purification methods, or metal recovery techniques tailored to their characteristics.
Solution purification
Solution purification is a crucial step in hydrometallurgical processes to remove impurities and contaminants from the leach solution obtained after the leaching step. Purification is necessary to obtain a solution enriched with the desired metal and to ensure the quality and purity of the final product. Various techniques can be employed for solution purification in hydrometallurgy.
Including:
1. Filtration: Filtration is a common method used to remove solid particles or suspended impurities from the leach solution. The solution is passed through a filter medium, such as filter presses, filter cloths, or filter papers, which retain the solid particles while allowing the clarified solution to pass through.
2. Solvent Extraction: Solvent extraction, also known as liquid-liquid extraction, is a widely used purification technique in hydrometallurgy. It involves the transfer of the metal ions from the aqueous leach solution to an organic phase by selective extraction. The organic phase, known as the extractant or solvent, contains specific reagents that preferentially bind to the metal ions, allowing their separation from the aqueous solution. After extraction, the loaded organic phase can be further processed to recover the metal or undergo stripping to transfer the metal ions back into an aqueous solution.
3. Ion Exchange: Ion exchange is another purification method used to remove impurities from the leach solution. It involves the exchange of metal ions in the solution with other ions present in the solid resin or exchange material. The resin selectively adsorbs the unwanted metal ions while allowing the desired metal ions to remain in the solution. The loaded resin can then be regenerated or processed further to recover the metal ions.
4. Precipitation: Precipitation is employed to selectively remove specific metal ions from the leach solution by inducing their chemical reaction to form insoluble compounds. By adjusting the pH, and temperature, or adding specific reagents, the metal of interest can be precipitated as a solid compound while leaving behind other impurities in the solution. The precipitate can then be separated through filtration, sedimentation, or other solid-liquid separation techniques.
5. Electrochemical Methods: Electrochemical techniques, such as electrowinning or electrorefining, can be used for purification and metal recovery. Electrowinning involves passing an electric current through the leach solution, causing the metal ions to be reduced and plated onto a cathode, resulting in the deposition of the metal in a pure form. Electrorefining is a similar process used to purify impure metals obtained from other sources by selectively dissolving the impurities from an anode and depositing the purified metal on a cathode.
These are some of the common techniques employed for solution purification in hydrometallurgy. The choice of purification method depends on factors such as the nature of impurities, the desired metal product, the characteristics of the leach solution, and the overall process requirements.
What is Pyrometallurgy?
Pyrometallurgy is a branch of extractive metallurgy that involves high-temperature processes to extract and refine metals from their ores or concentrates. It relies on the application of heat and various chemical reactions in a solid state to separate and recover metals.
The ore or concentrate is typically subjected to several steps, including:
1. Ore Preparation: The ore is prepared by crushing, grinding, and sometimes additional steps such as roasting or drying. The goal is to reduce the ore size and create a suitable feed for the subsequent steps.
2. Roasting: Roasting is a process in which the ore is heated in the presence of air or oxygen-enriched gas. It is carried out at elevated temperatures but below the melting point of the metal. Roasting serves several purposes, such as removing volatile impurities, driving off moisture, oxidizing sulfide minerals, and promoting desirable chemical reactions.
3. Smelting: Smelting is a key step in pyrometallurgy where the roasted ore, along with fluxes (such as limestone or silica), is heated in a furnace. The high temperatures cause the ore to melt, and chemical reactions occur, leading to the separation of the desired metal from the gangue (unwanted materials). During smelting, the metal collects at the bottom of the furnace as a molten product known as matte or crude metal.
4. Refining: Refining involves further purification of the crude metal obtained from smelting to remove impurities and obtain a high-purity metal. Different strategies such as oxidation, diminishment, electrolysis, or chemical responses are utilized within the refining stage to realize the required virtue and quality of the metal.
Throughout the pyrometallurgical process, temperature control, chemical reactions, and careful management of the furnace atmosphere are crucial for efficient metal extraction and refining. Factors such as furnace design, heat source, reaction kinetics, and the nature of the ore determine the specific parameters and conditions employed in each step.
Pyrometallurgy is broadly utilized for the extraction and handling of different metals, counting press, copper, lead, zinc, nickel, tin, and valuable metals like gold and silver. It is particularly suitable for ores with high metal concentrations or ores that are not amenable to hydrometallurgical processes.
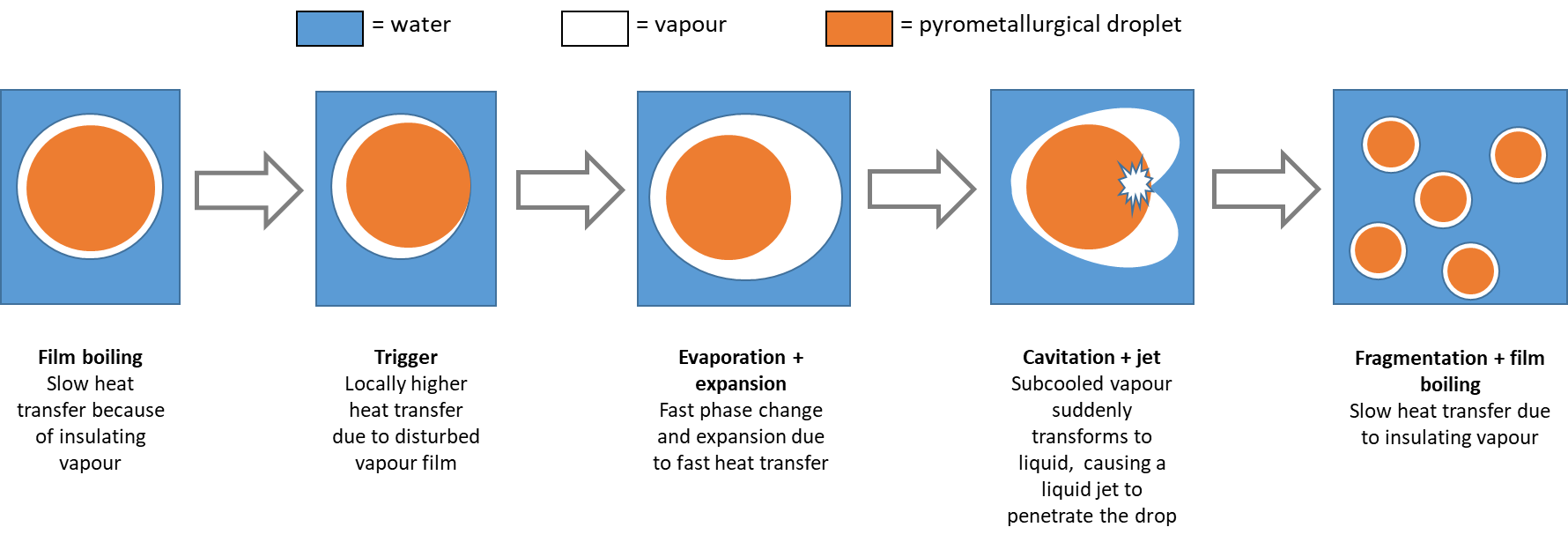
The choice of pyrometallurgical processes depends on factors such as the characteristics of the ore, the target metal, the desired product quality, and economic considerations. Different metals may require specific variations and adaptations of the pyrometallurgical steps to optimize metal recovery and meet the desired specifications.
Process description
The pyrometallurgical process typically involves the following steps:
1. Ore Preparation: The ore is crushed, ground, and sometimes subjected to additional pre-treatment processes like roasting or drying. This step aims to reduce the ore size, increase its surface area, and remove any moisture or volatile components.
2. Roasting: The ore is heated in the presence of air or oxygen-enriched gas at temperatures below the melting point of the metal. Roasting serves several purposes, including the removal of volatile impurities, conversion of certain minerals into more desirable forms, and oxidation of sulfide minerals. Roasting can improve the amenability of the ore for subsequent processing steps.
3. Smelting: Smelting involves heating the roasted ore along with fluxes (such as limestone or silica) in a furnace. The furnace is operated at high temperatures above the melting point of the metal. The smelting process promotes chemical reactions that result in the separation of the desired metal from the gangue materials. The fluxes help to form a slag, which acts as a liquid layer floating on top of the molten metal and helps to remove impurities by forming a separate phase.
4. Refining: The crude metal obtained from smelting, known as matte, undergoes further refining to remove impurities and achieve the desired purity and quality of the metal. Refining methods depend on the specific metal and can include processes like oxidation, reduction, electrolysis, or chemical reactions. These processes help to eliminate remaining impurities, adjust the composition of the metal, and obtain the desired product specifications.
5. Casting and Solidification: Once the metal is refined, it is cast into appropriate forms such as ingots, bars, or other shapes for further processing or commercial use. The molten metal is poured into molds and allowed to solidify. Controlled cooling rates and solidification processes can affect the final properties of the metal product.
It’s critical to note that the particular subtle elements and varieties of the pyrometallurgical preparation can change depending on the sort of mineral, the target metal, and the required conclusion item. Different metals and ores may require specific fluxes, temperature profiles, furnace designs, and refining techniques to optimize metal extraction, minimize impurities, and achieve the desired product quality.
Pyrometallurgical forms are broadly utilized in businesses such as press and steel generation, copper purifying, lead and zinc refining, and different other metal extraction and refining operations. The efficiency and success of the pyrometallurgical process depend on careful control of temperature, reaction conditions, and the management of various parameters to ensure effective metal extraction and quality control.
Advantages of pyrometallurgy
Pyrometallurgy offers several advantages as a metallurgical process:
1. Versatility: Pyrometallurgy applies to a wide range of metals and ores. It can be utilized for the extraction and refining of different metals such as press, copper, lead, zinc, nickel, tin, and valuable metals like gold and silver. The flexibility of pyrometallurgical processes allows for the treatment of different ore types and the recovery of multiple metals from complex ores.
2. High Metal Recovery: Pyrometallurgy is often capable of achieving high metal recovery rates. The high temperatures employed in the process promote efficient separation of the desired metal from the gangue materials in the ore. This results in a relatively high yield of the metal product.
3. Energy Efficiency: Pyrometallurgical processes can be energy-efficient, particularly when compared to other metallurgical techniques like hydrometallurgy. The high temperatures required for pyrometallurgy can often be obtained through the combustion of fuels or the utilization of waste heat from other industrial processes. Additionally, the exothermic nature of certain reactions within the process can help in reducing energy consumption.
4. Scalability: Pyrometallurgical processes are highly scalable, allowing for the treatment of large quantities of ore or concentrates. This makes it suitable for industrial-scale production and can result in economies of scale.
5. Synergistic Byproducts: Pyrometallurgical processes often generate valuable byproducts in addition to the main metal product. For example, in copper smelting, valuable byproducts like sulfuric acid, copper matte, and slag can be obtained. These byproducts can be sold or further processed, adding economic value to the overall process.
6. Process Flexibility: Pyrometallurgical processes can be adjusted and optimized for different ores, concentrates, and impurity levels. The process parameters, such as temperature, gas composition, and residence time, can be modified to accommodate variations in feed materials and achieve the desired metal quality and specifications.
7. Relatively Lower Water Consumption: Compared to hydrometallurgy, pyrometallurgical processes generally require less water for ore processing and metal recovery. This will be profitable in locales where water accessibility is restricted or in situations where water preservation could be a need.
8. Ability to Handle High-Grade Ores: Pyrometallurgy is particularly suitable for treating high-grade ores that contain a significant concentration of the desired metal. The high-temperature process can efficiently extract and separate the metal from such ores, resulting in a relatively high metal content in the final product.
It’s important to note that while pyrometallurgy offers various advantages, it may also have certain limitations. It may not be suitable for low-grade ores or ores with complex compositions that require more specialized processing techniques. Pyrometallurgical processes can generate emissions and waste products that need to be carefully managed to minimize their environmental impact.
Comparison Chart
Sure! Here’s a comparison chart highlighting the key differences between hydrometallurgy and pyrometallurgy:
| Hydrometallurgy | Pyrometallurgy |
|---|---|
| Uses aqueous solutions or liquid solvents for metal extraction | Relies on high-temperature processes and solid-state reactions |
| Low temperatures, below the boiling point of water | High temperatures, often above the melting point of metals |
| Chemical reactions in the liquid phase | Chemical reactions in solid-state |
| Generally low-energy heat sources | Requires substantial energy for high-temperature processing |
| Suitable for low-grade ores and metals amenable to dissolution | Suitable for high-grade ores and metals requiring high heat |
| Typically lower environmental impact | Higher potential for emissions and waste generation |
| Generally more energy-efficient | May require significant energy input |
| Lower metal recovery compared to pyrometallurgy | Higher metal recovery rates |
| Can generate valuable byproducts | Can generate valuable byproducts and slag |
| Can be adapted for different ores and impurity levels | Can be adapted for different ores and impurity levels |
Please note that this chart provides a general overview, and specific processes within hydrometallurgy and pyrometallurgy may vary in terms of the parameters and conditions used.
Future Trends and Developments
Future trends and developments in hydrometallurgy and pyrometallurgy are expected to focus on several key areas:
1. Sustainability and Environmental Impact: The metallurgical industry is increasingly prioritizing sustainable practices and reducing its environmental footprint. Future improvements will likely include the advancement of more naturally inviting forms, such as the utilization of renewable vitality sources, progressed squander administration, and the improvement of greener reagents and advances to play down outflows and squander era.
2. Energy Efficiency: Enhancing energy efficiency in both hydrometallurgy and pyrometallurgy will be a significant area of focus. This can involve the development of more efficient heat transfer systems, advanced process control technologies, and the utilization of waste heat and energy recovery methods to reduce overall energy consumption in metal extraction and refining.
3. Process Optimization and Integration: Advances in modeling, simulation, and optimization techniques will enable more efficient and effective process design and operation. Integrated approaches that combine hydrometallurgical and pyrometallurgical processes, as well as the integration of different unit operations, can lead to improved metal recovery, reduced energy requirements, and enhanced overall process performance.
4. Selective Extraction and Recovery: Future developments will focus on the development of innovative and selective extraction methods to improve metal recovery from complex ores, low-grade deposits, and secondary resources. Advanced separation techniques, such as selective leaching, solvent extraction, and ion exchange, will continue to be refined to achieve higher metal purity and minimize the extraction of unwanted elements.
5. Advanced Control Systems and Automation: The implementation of advanced control systems, artificial intelligence, and automation technologies will enhance process monitoring, control, and optimization. Real-time information investigation, machine learning calculations, and prescient modeling will offer assistance in progress handling productivity, decrease inconstancy, and empower proactive decision-making for way better-prepared results.
6. Alternative Raw Materials: As traditional ore deposits become depleted, there will be a growing focus on developing and utilizing alternative raw materials. This includes the exploration and development of new sources of ores, such as polymetallic nodules, deep-sea mining, and urban mining of electronic waste. These alternative sources may require innovative processing techniques and specialized extraction methods.
7. Circular Economy Approaches: The concept of a circular economy, where materials are reused, recycled, and recovered, will have an increasing influence on metallurgical processes. Future developments will explore strategies for the recovery of valuable metals from secondary sources, including industrial byproducts, electronic waste, and mine tailings, to minimize waste and maximize resource efficiency.
8. Advanced Analytical Techniques: Advances in analytical techniques, such as spectroscopy, microscopy, and sensor technologies, will enable more precise monitoring and characterization of process streams, leading to improved process control, a better understanding of reaction mechanisms, and enhanced quality control of the final metal products.
These future trends and developments aim to address challenges related to sustainability, energy efficiency, resource scarcity, and environmental impact while optimizing metal extraction, refining processes, and product quality in both hydrometallurgy and pyrometallurgy. The extreme objective is to make more productive, ecologically neighborly, and financially practical metallurgical forms that meet the requests of a quickly advancing worldwide industry.
Conclusion
The difference between hydrometallurgy and pyrometallurgy lies in their fundamental principles, temperature ranges, and applications. Hydrometallurgy focuses on the use of aqueous solutions for metal extraction at lower temperatures, making it suitable for low-grade ores. Employs high-temperature processes to extract metals from high-grade ores. Both processes contribute significantly to the extraction of essential metals, playing crucial roles in various industries.
By understanding the subtleties of hydrometallurgy and pyrometallurgy, experts within the field of metallurgy can make informed choices around the foremost reasonable strategy for a particular metal or mineral. As headways proceed in both forms, the journey for more proficient, economical, and environmentally friendly extraction strategies remains a driving constraint within the metallurgical industry.
