Reactions are at the heart of understanding how substances interact with each other. Two common types of reactions that occur are synthesis reactions and dissociation reactions. Whereas they may sound comparative, there are crucial contrasts between the two. We will delve into the details of synthesis and dissociation reactions, exploring their definitions, mechanisms, and real-world examples. By the end, you’ll have a clear understanding of the disparities and be able to differentiate between these essential chemical processes.
Importance of understanding different types of reactions
Understanding different types of reactions is crucial for several reasons:
1. Prediction of chemical behavior: Knowledge of different reaction types allows chemists to predict how different substances will behave when they come into contact with each other. This understanding is fundamental for designing and controlling chemical processes.
2. Reaction optimization: Different types of reactions require different conditions, such as temperature, pressure, and catalysts, for optimal performance. By understanding the specific requirements of each reaction type, chemists can optimize reaction conditions to achieve desired outcomes efficiently.
3. Reaction mechanism elucidation: Understanding different reaction types helps in unraveling the underlying mechanisms by which chemical transformations occur. This knowledge enables scientists to propose and validate reaction mechanisms, leading to a deeper understanding of chemical processes.
4. Synthesis of new compounds: Different types of reactions are employed to synthesize new compounds. Understanding these reactions allows chemists to design synthetic routes and choose appropriate reactants to produce desired products efficiently. This can be fundamental in medicate revelation, fabric science, and other areas where the blend of modern compounds is pivotal.
5. Safety considerations: Different reaction types may pose different safety risks. Understanding the characteristics of each reaction type helps in assessing potential hazards and implementing appropriate safety measures in laboratory or industrial settings. This knowledge ensures the protection of researchers, technicians, and the environment.
6. Environmental impact: By understanding different reaction types, chemists can develop more sustainable and environmentally friendly processes. They can choose reactions that minimize waste generation, reduce energy consumption, and utilize greener solvents, thus contributing to the principles of green chemistry.
7. Education and scientific communication: Understanding different types of reactions is fundamental for effective communication and collaboration among scientists. It allows researchers to accurately describe and discuss chemical transformations, facilitating the sharing of knowledge and advancements in the field.
Understanding different types of reactions is essential for predicting chemical behavior, optimizing reactions, elucidating reaction mechanisms, synthesizing new compounds, ensuring safety, minimizing environmental impact, and promoting scientific collaboration and communication. It forms the foundation for advancements in chemistry and related fields, contributing to scientific progress and practical applications.
Definition of Synthesis Reaction and Dissociation Reaction
Synthesis Reaction: A synthesis reaction, too known as a combination or composition response, could be a sort of chemical response in which two or more substances combine to create a single, more complex item. It is the process of combining simpler reactants to produce a more complex compound. Synthesis reactions are characterized by the common condition organized: A + B → AB, where A and B are the reactants, and AB is the item. Energy may be either absorbed or released during a synthesis reaction, and catalysts may be used to facilitate the reaction. Synthesis reactions play a crucial part in different characteristic, mechanical, and research facility forms, counting the arrangement of compounds and the blend of imperative chemicals.
Dissociation Reaction: A dissociation reaction, too known as a decay response, maybe a sort of chemical response in which a single compound breaks down into two or easier substances, either components or littler compounds. Dissociation reactions typically occur when a compound is exposed to certain conditions such as heat, electricity, or a solvent. They are characterized by the general equation format: AB → A + B, where AB is the reactant compound, and A and B are the products. Dissociation reactions often require an input of energy to break the chemical bonds within the compound. Factors such as temperature, pressure, and solvent properties influence the extent of dissociation.
Dissociation reactions are significant in various contexts, such as the electrolysis of water, the dissociation of acids and bases, and the understanding of chemical equilibrium. These definitions give an essential understanding of synthesis reactions, where easier reactants combine to create a more complex item, and separation reactions, where a compound breaks down into less difficult substances.
What are Synthesis Reactions?
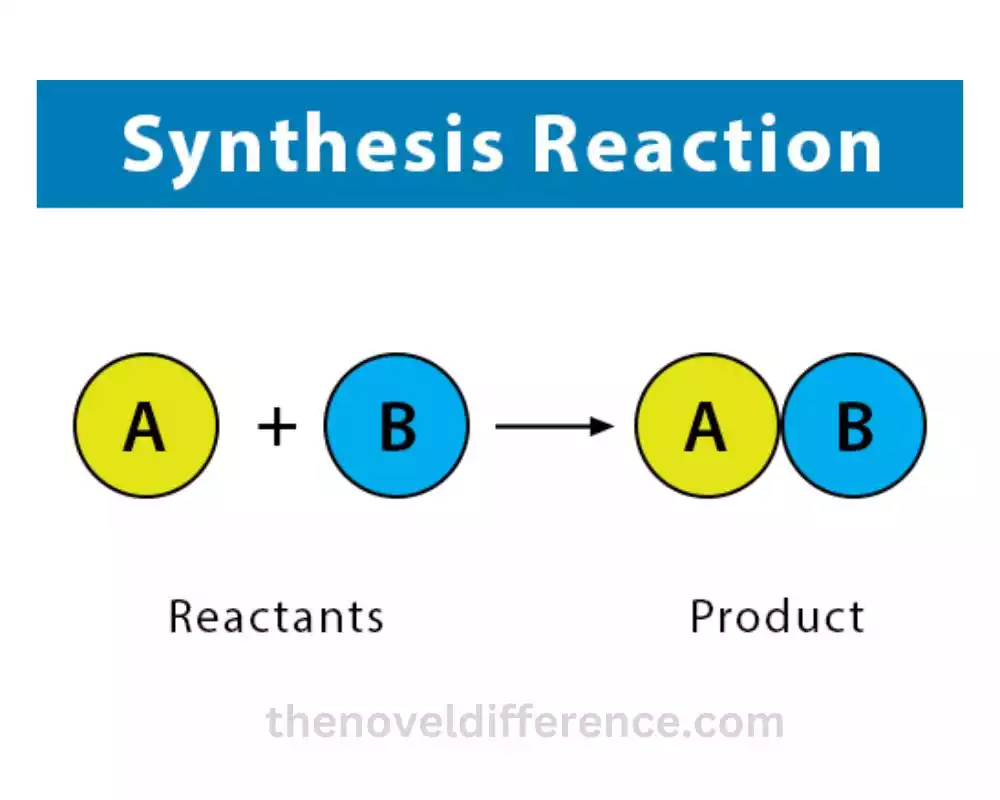
Synthesis reactions, also known as combination reactions or composition reactions, are a type of chemical reaction in which two or more substances combine to form a single, more complex product. The reactants come together and bond to create a new compound or molecule.
The general equation format for a synthesis reaction is:
A + B → AB
A and B represent the reactants, which can be elements, compounds, or ions. AB represents the product, which is a compound formed by the combination of A and B.
Synthesis reactions can happen in different areas of chemistry, counting natural chemistry, inorganic chemistry, and organic chemistry. They play a crucial part in the arrangement of new compounds and are basic for the synthesis of different substances, extending from basic particles to complex polymers.
Synthesis reactions can include diverse sorts of chemical holdings, such as covalent bonds, ionic bonds, or metallic bonds, depending on the nature of the reactants and the coming-about item. These responses can be impacted by variables such as temperature, weight, concentration, and the nearness of catalysts.
An illustration of a synthesis reaction is the combination of hydrogen gas (H2) and oxygen gas (O2) to create water (H2O):
2H2 + O2 → 2H2O
The elements hydrogen and oxygen combine to produce the compound water.
Synthesis reactions are of great importance in various scientific and industrial applications. They are utilized within the union of pharmaceuticals, polymers, fertilizers, colors, and numerous other compounds. Understanding synthesis reactions allows chemists to design efficient synthetic routes and manipulate chemical reactions to obtain desired products.
Characteristics and key features
Synthesis reactions possess several characteristics and key features that distinguish them from other types of chemical reactions.
Some of these characteristics and key features include:
1. Combination of substances: Synthesis reactions involve the combination of two or more substances, which can be elements, compounds, or ions, to form a single product. The reactants come together and bond to create a more complex compound or molecule.
2. Single product formation: Unlike other types of reactions that produce multiple products, synthesis reactions typically yield a single product. The reactants combine to form a new compound, represented by the general equation format A + B → AB.
3. Energy changes: Synthesis reactions can either release energy (exothermic) or absorb energy (endothermic). The energy change depends on the nature of the reactants and products involved. Exothermic synthesis reactions release energy, often in the form of heat, while endothermic synthesis reactions absorb energy from the surroundings.
4. Catalysts: Catalysts are substances that can speed up the rate of a chemical reaction without being consumed in the process. Synthesis reactions may employ catalysts to enhance reaction rates and lower the activation energy required for the reaction to occur. Catalysts provide an alternative reaction pathway, making the synthesis process more efficient.
5. Bond formation: Synthesis reactions involve the formation of chemical bonds between atoms or ions. The reactants’ existing bonds are broken, and new bonds are formed to create the product. The type of bonding depends on the nature of the reactants and the resulting compound.
6. Reaction conditions: The conditions under which a synthesis reaction occurs can significantly influence the reaction rate and product formation. Factors such as temperature, weight, concentration, and the nearness of a catalyst can influence the result of the response.
7. Importance and applications: Synthesis reactions are of immense importance in various scientific and industrial applications. They play an imperative part in natural synthesis, medicate revelation, materials science, fabricating forms, and numerous other areas. Understanding the characteristics of synthesis reactions allows chemists to design efficient synthetic routes and manipulate reactions to obtain desired products.
By understanding these characteristics and key features, chemists can predict and control the behavior of synthesis reactions, enabling them to design and optimize chemical processes effectively.
General equation format
The general equation format for a synthesis reaction is:
A + B → AB
“A” and “B” speak to the reactants included within the synthesis reaction, and “AB” speaks to the coming of the item. The plus sign (+) indicates that the reactants are combining or coming together to form the product.
It’s important to note that the reactants and products in a synthesis reaction can vary widely depending on the specific chemical reaction being described. The reactants can be elements, compounds, or ions, and the product is a compound formed by the combination of the reactants.
Here are a few examples illustrating the general equation format for synthesis reactions:
1. Formation of water: 2H2 + O2 → 2H2O In this reaction, hydrogen gas (H2) and oxygen gas (O2) combine to form water (H2O).
2. Formation of sodium chloride: Na + Cl2 → 2NaCl Here, sodium (Na) and chlorine (Cl2) react to form sodium chloride (NaCl).
3. Formation of calcium oxide: Ca + O2 → CaO In this reaction, calcium (Ca) reacts with oxygen gas (O2) to produce calcium oxide (CaO).
The general equation format provides a basic framework for understanding how reactants combine to form a product in a synthesis reaction. It is important to consult specific reaction equations and conditions to fully understand the details of individual synthesis reactions.
What is Dissociation Reactions?
Separation responses, moreover known as deterioration responses, allude to a sort of chemical response in which a single compound breaks down into two or easier substances. The reactant compound undergoes a chemical change, resulting in the separation of its constituent elements or smaller compounds.
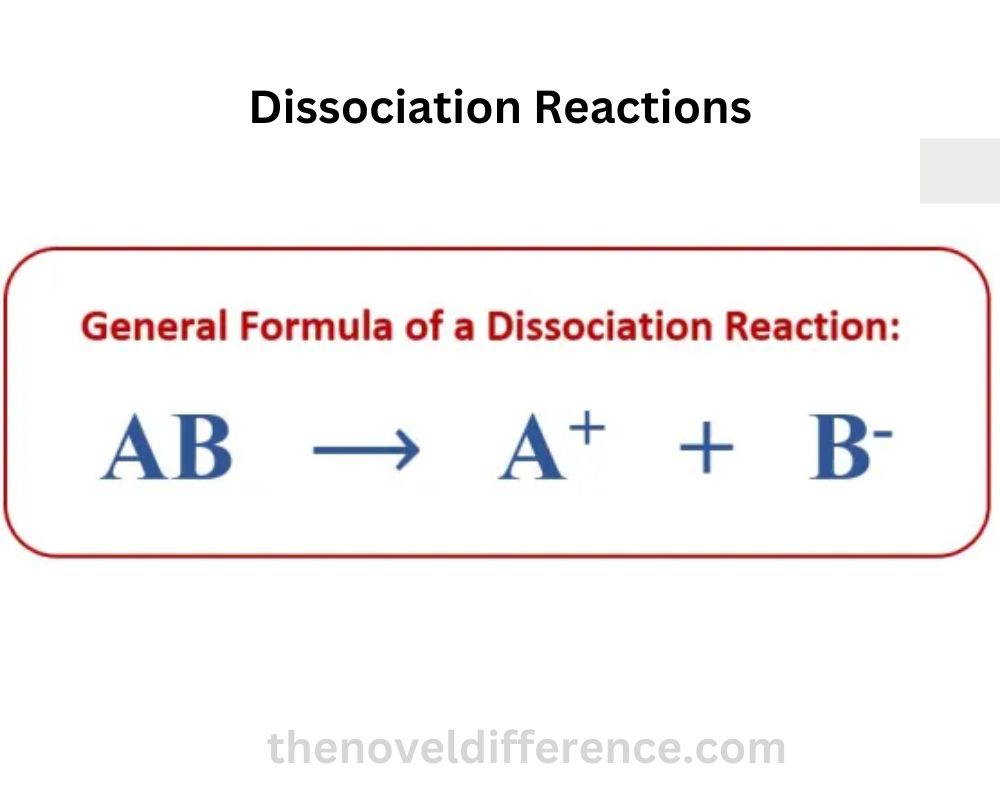
The general equation format for a dissociation reaction is:
AB → A + B
“AB” speaks to the reactant compound, and “A” and “B” speak to the items of the separation response. The reactant compound breaks apart, and its components are released as separate entities.
Dissociation reactions can occur through various mechanisms and under different conditions. Some common types of dissociation reactions include:
1. Ionic compound dissociation: Dissociation reactions commonly occur with ionic compounds in aqueous solutions. The compound dissociates into its constituent ions when dissolved in a suitable solvent. For example NaCl → Na+ + Cl- In this response, sodium chloride (NaCl) dissociates into sodium particles (Na+) and chloride particles (Cl-) in a watery arrangement.
2. Acid-base dissociation: Many acids and bases undergo dissociation reactions when dissolved in water. The acid or base dissociates into its respective ions. HCl → H+ + Cl- In this reaction, hydrogen chloride (HCl) dissociates into hydrogen ions (H+) and chloride ions (Cl-) when dissolved in water.
Dissociation reactions are often influenced by factors such as temperature, pressure, and solvent properties. Higher temperatures and increased pressure can promote dissociation, while specific solvents can facilitate the dissociation process.
Dissociation reactions play a significant role in various areas of chemistry. They are crucial for understanding acid-base reactions, electrolysis, chemical equilibrium, and the behavior of electrolytes in solution. Dissociation reactions also have practical applications in fields such as electrochemistry, industrial processes, and the study of chemical equilibrium in environmental systems.
It’s vital to note that not all compounds experience separation responses. The inclination to separate depends on the chemical nature of the compound and the conditions beneath which the response takes put.
Factors influencing dissociation
Dissociation reactions can be influenced by several factors that affect the extent or rate at which a compound dissociates.
The key factors influencing dissociation include:
1. Temperature: Temperature plays a significant role in dissociation reactions. Generally, increasing the temperature enhances the rate of dissociation. Higher temperatures provide more energy to break the chemical bonds within the compound, promoting the separation of its components. However, there may be exceptions where higher temperatures can favor the reverse reaction or decrease dissociation, depending on the specific reaction and its thermodynamics.
2. Pressure: Pressure can influence dissociation reactions, particularly in cases where the reactant compound is in the gas phase. Expanding the weight can lead to the next concentration of reactant particles, which in turn can advance separation. Pressure effects are more pronounced for gas-phase reactions and may have limited influence on dissociation in solutions.
3. Solvent properties: The properties of the solvent in which a dissociation reaction takes place can impact the extent of dissociation. Solvents can stabilize or destabilize ions or compounds, affecting their tendency to dissociate. For example, polar solvents often enhance dissociation by solvating ions and weakening their attractive forces, facilitating the separation of components.
4. Concentration: The concentration of the reactant compound can influence dissociation. Higher concentrations generally increase the likelihood of collisions between molecules, thereby promoting dissociation. However, there may be cases where the concentration effect on dissociation is more complex and depends on the specific reaction and its equilibrium constants.
5. Presence of catalysts: Catalysts are substances that can accelerate the rate of a chemical reaction without being consumed in the process. In dissociation reactions, catalysts can lower the activation energy required for the reaction to occur, facilitating the breaking of chemical bonds and promoting dissociation.
6. Nature of the compound: The chemical composition and structure of the compound undergoing dissociation play a crucial role. Factors such as bond strength, polarity, and intermolecular forces can influence the ease with which a compound dissociates. Compounds with weaker bonds or weaker intermolecular forces tend to undergo dissociation more readily.
It is vital to note that the impact of these variables on separation responses may change depending on the particular response and the nature of the compound included. Understanding these factors helps in predicting and manipulating dissociation reactions, contributing to the design and optimization of chemical processes.
Solvent properties
Solvent properties refer to the characteristics and behaviors of a substance that is capable of dissolving other substances to form a solution. Solvents play a crucial role in many chemical processes, including dissolution, reactions, and separation techniques. The properties of a solvent can significantly influence the behavior of solutes and the overall chemistry of a system.
Some important solvent properties include:
1. Polarity: Solvents can be classified as polar or nonpolar based on their molecular polarity. Polar solvents have an uneven distribution of electron density and possess a positive and negative pole within the molecule. Illustrations of polar solvents incorporate water, alcohol, and acetone. Nonpolar solvents, on the other hand, have a relatively even distribution of electron density. Illustrations of nonpolar solvents incorporate hexane, benzene, and toluene. The extremity of dissolvable influences solute-solvent intuitive, with polar solvents favoring the disintegration of polar solutes and nonpolar solvents favoring nonpolar solutes.
2. Dielectric constant: The dielectric constant is a measure of a solvent’s ability to reduce the electrostatic forces between charged particles. It indicates the solvent’s ability to dissolve ions or polar substances. Solvents with high dielectric constants, such as water, have a greater ability to dissolve ionic compounds and facilitate dissociation reactions. Nonpolar solvents typically have lower dielectric constants.
3. Solubility: Solubility is the ability of a solute to dissolve in a given solvent. Solvent properties, such as polarity and molecular interactions, influence solubility. Polar solvents break up polar or ionic solutes, whereas nonpolar solvents break down nonpolar solutes. The solubility of a solute in a particular solvent can vary widely, and it is essential to choose a suitable solvent to ensure effective dissolution and desired outcomes in chemical processes.
4. Boiling point and volatility: The boiling point of a solvent is the temperature at which it changes from a liquid to a gas phase. Solvents with higher boiling points generally require higher temperatures for evaporation. Volatility refers to a solvent’s tendency to evaporate. Solvents with higher volatility evaporate more quickly. These properties are important considerations in processes such as distillation, where the separation of solvents from solutes is desired.
5. Miscibility: Miscibility refers to the ability of two or more solvents to mix and form a homogeneous solution. Solvent miscibility depends on their relative polarities and interactions. Polar solvents are by and large miscible with other polar solvents, whereas nonpolar solvents are miscible with other nonpolar solvents. The miscibility of solvents can be exploited to create solvent blends with specific properties and solvation abilities.
6. Toxicity and environmental impact: Solvent choice also involves considering the toxicity and environmental impact of the solvent. A few solvents can be destructive to human well-being or have antagonistic impacts on the environment. Green solvents, such as water or certain natural solvents determined from renewable assets, are picking up significance due to their decreased harmfulness and natural impression.
The selection of a solvent with suitable properties is crucial for achieving desired results in chemical processes. Understanding solvent properties helps chemists make informed decisions about solvents, ensuring efficient dissolution, reaction conditions, and separation techniques.
Difference between Synthesis and Dissociation Reactions
The main difference between synthesis reactions and dissociation reactions lies in the transformation of compounds.
Synthesis Reactions:
1. Definition: Synthesis reactions, moreover known as combination or composition responses, include the combination of two or more substances to make a more complex compound.
2. Reactants: Synthesis reactions start with simpler reactants, which can be elements, compounds, or ions.
3. Product Formation: The reactants chemically bond together to form a single, more complex product.
4. General Equation Format: The general equation format for a synthesis reaction is A + B → AB, where A and B are the reactants, and AB is the product.
5. Energy Change: Synthesis reactions can be exothermic (release energy) or endothermic (absorb energy), depending on the specific reaction.
6. Catalysts: Catalysts may or may not be involved in synthesis reactions, depending on the reaction’s requirements.
7. Importance: Synthesis reactions are essential in various natural, industrial, and laboratory processes. They are crucial for the formation of compounds and the synthesis of important chemicals.
Dissociation Reactions:
1. Definition: Dissociation reactions, also known as decomposition reactions, involve the breakdown of a single compound into two or more simpler substances.
2. Reactant: Dissociation reactions start with a compound as the reactant.
3. Product Formation: The reactant compound breaks apart, and its components (atoms, ions, or smaller compounds) are released as separate entities.
4. General Equation Format: The general equation format for a dissociation reaction is AB → A + B, where AB is the reactant compound, and A and B are the products.
5. Energy Change: Dissociation reactions often require an input of energy to break the chemical bonds within the compound.
6. Factors Influencing Dissociation: Factors such as temperature, pressure, solvent properties, and the nature of the compound influence the extent of dissociation.
7. Importance: Dissociation reactions play a significant role in various contexts, including the electrolysis of water, the dissociation of acids and bases, and the understanding of chemical equilibrium.
Synthesis reactions include the combination of easier reactants to create a more complex item, whereas separation responses include the breakdown of a compound into simpler substances. Synthesis reactions result within the arrangement of a single item, whereas separation responses surrender different items.
Comparison Chart
Here’s a comparison chart highlighting the key differences between synthesis reactions and dissociation reactions:
| Synthesis Reactions | Dissociation Reactions |
|---|---|
| Combination of two or more substances to form a more complex compound | Breakdown of a compound into simpler substances |
| Simpler reactants (elements, compounds, ions) | A compound as the reactant |
| Formation of a single, more complex product | Formation of multiple products |
| A + B → AB | AB → A + B |
| Can be exothermic or endothermic | Often requires an input of energy |
| May or may not involve catalysts | Catalysts may or may not be involved |
| Reaction conditions such as temperature, pressure, concentration, presence of a catalyst | Temperature, pressure, solvent properties, nature of the compound |
| Important in natural, industrial, and laboratory processes | Relevant in various contexts such as electrolysis, acid-base reactions, chemical equilibrium |
Remember that while this comparison chart provides a general overview of the differences between synthesis reactions and dissociation reactions, individual reactions may have unique characteristics or factors that can influence their behavior.
Conclusion
Synthesis and Dissociation reactions are essential chemical processes with distinct characteristics. Synthesis reactions include the combination of substances to create more complex compounds, whereas separation responses include the breaking down of compounds into less complex substances.
Understanding the disparities between these two types of reactions is crucial in comprehending various chemical phenomena, from natural processes to laboratory experiments. By grasping the fundamentals, you can navigate the world of chemistry with confidence.
