Introduction to Electron and Beta Particle
Beta particles and electrons are fundamental subatomic particles that have a major role in the structure and behavior of matter. Electrons are positively charged particles that are found inside the electron cloud of molecules. They play a key role in chemical reactions and electricity.
Beta particles On the contrary could be electrons (beta-minus) or positrons (beta-plus) which are released in certain radiation decay. Understanding the differences and similarities is crucial for all areas of science and technology.
Characteristics of Electrons
- Subatomic Particle: Electrons belong to the subatomic particle. This means their size is smaller than that of atoms, and forms the electron cloud that surrounds an atom.
- Negative Charge: Electrons have an electric charge that is negative with an approximate charge of 1 elemental charge (approximately -1.602 10-19 Coulombs).
- Mass: Electrons are of extremely low mass when compared to neutrons and protons. Their mass is approximately 1/1836th of the proton’s mass which is around 9.11 1031 kg.
- Position within the Atom: Electrons are present in various energies or electron shells that surround the nucleus of the atom. They are located in particular orbitals inside the shells.
- Function within Chemical Reactions: Electrons are involved in chemical bonding as well as reactions. They determine the chemical properties and play an important part in the formation of the chemical bonds that bind atoms to create compounds and molecules.
- History and Discovery: Electrons were discovered by J.J. Thomson in 1897 using his cathode-ray tube experiments. The discovery changed our understanding of the structure of atoms.
- Wave-Particle Duality: Electrons exhibit wave-like and particle-like properties this is known as the duality of wave particles, defined by quantum mechanics.
- Quantized Energy Levels: Electrons can only be found in certain quantized energy levels within an atom. The energy levels are characterized by quantum numbers and are correlated to particular orbitals.
- Spin: Electrons have intrinsic spin or angular momentum, that could have two possible values of (+1/2) or -1/2. The quantum number Spin can be that are used to describe the properties of electrons.
- Electron Cloud: Electrons are typically described as creating an electron cloud over the nucleus. This represents the possibility of locating an electron in a certain part of an atom.
- Electric Current: Electrons’ movement creates an electric current. In materials that conduct electricity, like metals, electrons flow freely, allowing the transfer of electricity.
- Valence Electrons: Electrons in the electron’s outer shell are called”valence electrons. These electrons are accountable for the chemical reactivity in an element and bonding abilities.
- Electrical Charge Conservation: The electrons have to conserve charge. In any physical or chemical process, the charge remains constant. This means that electrons cannot be made or destroyed, they simply travel between the atoms.
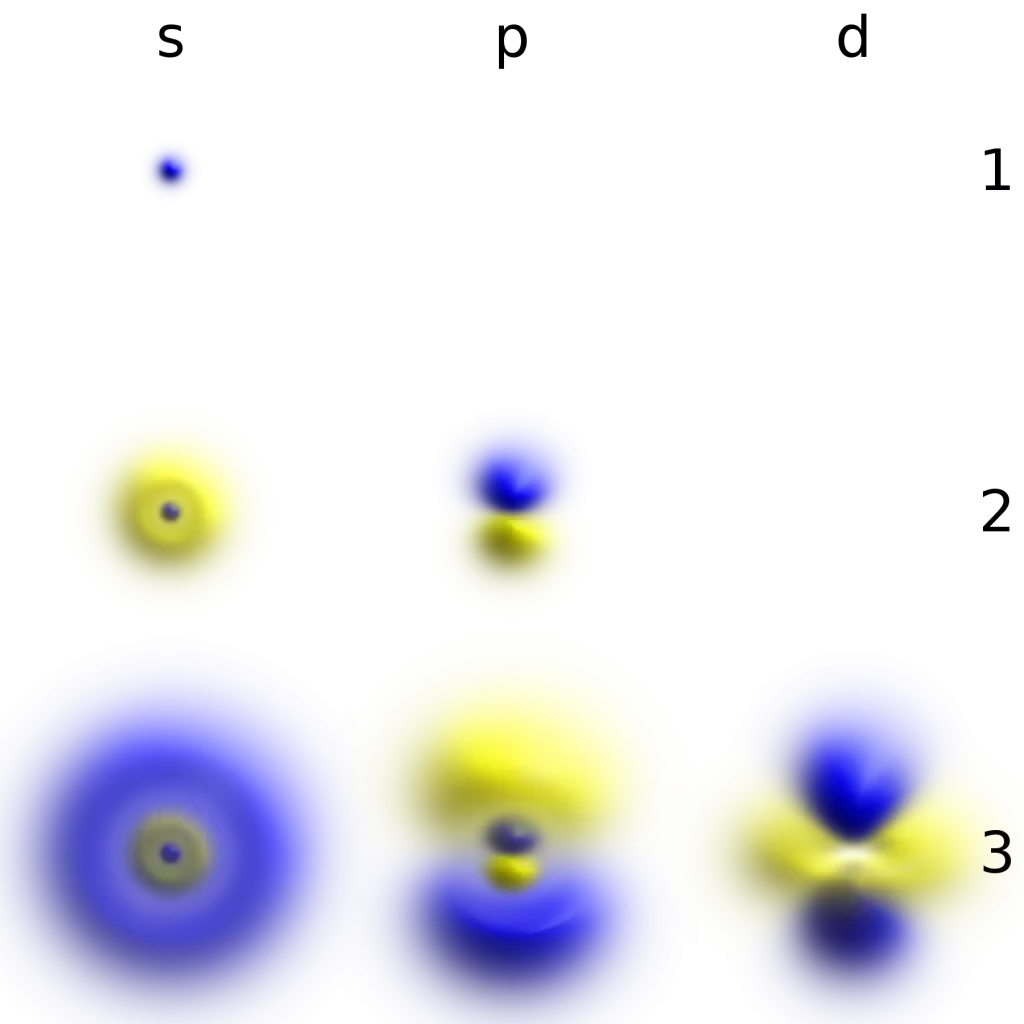
Characteristics of Beta Particles
- Subatomic Particles: The beta particles belong to the subatomic category specifically related to specific types of radioactive decay.
- The nature of beta particles: Beta particles can be electrons (beta-minus or B-) or Postrons (beta-plus, beta-plus,). Alpha-minus particles can also be described as electrons while beta-plus particles are also positrons.
- charge: The beta-minus particles (electrons) have a negative electrical charge, whereas the beta-plus particles (positrons) have positive charges. Beta-minus particles are charged with around 1 elementary charge, while beta-plus particles carry an elementary charge of +1 charge.
- mass: The beta particles possess an equivalent mass to electrons. Beta-minus particles weigh a weight of about 9.11 x 1031 kilograms and beta-plus particles carry a similar mass. This is a lot less than the mass of neutrons or protons.
- Emission from Nuclei: Beta particles are released from the nuclei of atomic atoms during a specific radiation-induced decay process. Beta-minus decay is the process of converting a neutron into a proton an electron (beta-minus particle) and an antineutrino. Beta-plus decay is the conversion of a proton to a neutron or proton, a positron (beta-plus particle) as well as the neutrino.
- The types of decay: Beta-plus decay, as well as decay that is beta-minus are two types of beta decay. Beta-minus decay is a more popular form of beta decay while beta-plus decay is more prevalent and can occur in particular situations, like some kinds of nuclei that are unstable.
- Kinetic Energy: The beta particle emits kinetic energy and their energy can differ based on the particular radioactive isotope in decay. The energy is divided among the beta particle and neutrino (in the beta-minus decay) or neutrino (in beta-plus decay).
- Penetrating Ability: Beta particles have a higher permeability than alpha particles, but they are less effective than Gamma Rays. They can be prevented by materials such as glass, plastic, or just a few millimeters of aluminum.
- interaction with matter: Beta particles ionize matter when they move through it, which makes them beneficial in applications such as radiation therapy to treat cancer.
- Rolle within Nuclear Reactions: Beta decay processes play an essential part in altering the composition of nuclear nuclei which leads to the transformation of one element to another, and contributes to that process known as nucleosynthesis within stars.
- Detection: Beta particles can be detected and measured with different instruments, including Geiger-Muller counters as well as scintillation detectors.
- Antiparticle Counterpart: The beta particle (electrons and the positrons) are antiparticles (antielectrons or the positrons). When the beta particle (positron) meets with an electron smash each other, turning their mass into energy through gamma radiation.
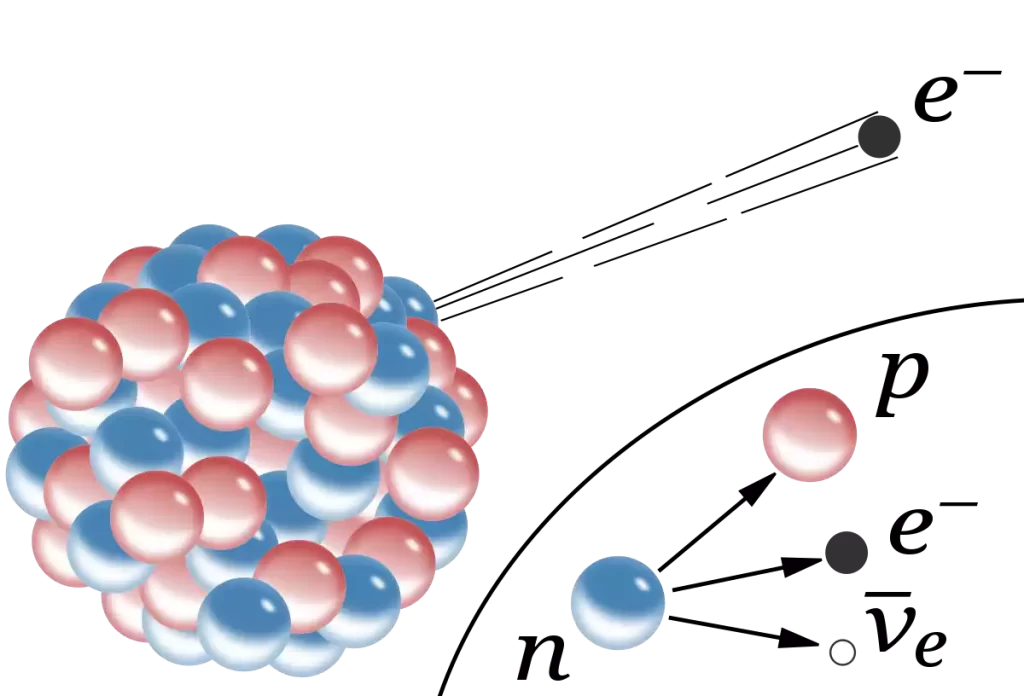
Electrons and Beta Particles in the comparison chart
Here’s a comparison chart outlining the key characteristics of electrons and beta particles:
| Characteristic | Electrons | Beta Particles |
|---|---|---|
| Subatomic Particle | Yes | Yes |
| Charge | Negative (-1 elementary charge) | Negative (Beta-minus, or -1) or Positive (Beta-plus, or +1) |
| Mass | Approximately 9.11 x 10^-31 kg | Approximately 9.11 x 10^-31 kg |
| Origin | Electron cloud of atoms | Emitted during beta decay from atomic nuclei |
| Location within an Atom | Electron shells | Nucleus (in the case of beta particles emitted during decay) |
| Role in Chemical Reactions | Determine chemical properties, participate in chemical bonding | Not directly involved in chemical reactions |
| Discovery and History | Discovered by J.J. Thomson in 1897 | Beta decay processes studied in the early 20th century |
| Nature of Particle | Electrons are always electrons | Beta particles can be electrons (beta-minus) or positrons (beta-plus) |
| Kinetic Energy | Varied energy levels | Emitted with kinetic energy during decay |
| Penetrating Ability | Limited penetration | Intermediate penetration can be stopped by materials like plastic |
| Interaction with Matter | Ionize matter as it passes through | Ionize matter as it passes through |
| Detection Methods | Detected using electron detectors | Detected using radiation detectors, such as Geiger-Muller counters |
| Interaction with Antiparticles | Electrons have no antiparticles | Beta-plus particles (positrons) have antiparticles (antielectrons) |
This chart highlights the key differences and similarities between electrons and beta particles, particularly in terms of their charges, masses, roles, and interaction with matter.
Significance of Understanding the Differences
Understanding the difference between beta particles and electrons is crucial in a variety of technological, scientific, and everyday situations:
- Atomic Structure: Understanding electrons is essential to understand the structure of the atoms. This is because it’s the way electrons are placed inside electron shells that determines the chemical properties of an element and its chemical reaction reactivity.
- Radioactive Decay: Knowing the beta particle is essential in the field of nuclear physics because beta decay processes are vital to explain the transformation of one substance into another. This information is essential in understanding the behavior of unstable nuclei as well as for the use of radiometric dates.
- Particle Physics: Electrons and beta particles are elementary particles that play a crucial role in understanding particle Physics. They are integral to the traditional particle physics model and are vital to understanding the basic elements that make up the Universe.
- Technologies: Electrons play a crucial role in electrical and electronic circuits. Knowing their characteristics is essential to the development as well as operation of electronics, ranging from microchips to energy generation.
- Radiation Therapy: For medical settings, the understanding of beta particles is essential in radiation therapy, as beta particles with high energy are utilized for treating cancerous tumors.
- Particle Detectors: The understanding of the beta particle’s behavior is crucial to designing and using particle detectors for experiments, like those carried out in particle accelerators and nuclear research facilities as well as medical imaging equipment such as PET scanners.
- Energy Production: Knowing the properties of electrons is crucial to energy production and distribution because they control the movement of electricity through the conductive materials, as well as energy generation as well as transmission.
- Environmental Monitoring: The information about beta particles is essential in assessing and monitoring the levels of radiation in the environment and possible dangers, and also the safety of nuclear power plants.
- Quantum Mechanics: Both electrons and beta particles play an important part in illustrating the fundamental principles of quantum mechanics, including the duality of wave particles, quantized energy levels, and preservation of energy.
- Space Exploration: Understanding subatomic particles such as electrons is essential in space exploration as well as the development of technology for spacecraft, satellites, or interplanetary space missions.
Understanding the difference between beta particles and electrons is vital across many scientific disciplines. It also has practical applications in the fields of technology as well as healthcare, energy, and fundamental research.
Similarities between Electrons and Beta Particles
Electrons and beta particles have many similarities, despite different charges and their origins. Here are a few most important similarities between them:
- Subatomic Particles: Both beta and electrons are subatomic particles. This is to say that they are less massive than atoms, and have a major role to play in the way matter behaves.
- Mass: Beta particles and electrons have almost identical masses. An electron’s mass equals around 9.11 1031 kilograms while beta particles (both Beta-minus as well as beta-plus) have masses that are similar to that.
- Wave-Particle Duality: Beta particles and electrons each exhibit a duality of wave-particles, an idea that is part of quantum mechanics. They behave as waves and particles, based on the conditions under which they are tested.
- Interactive in Electromagnetic Fields: Both beta particles and electrons interact in electromagnetic fields making them sensitive to electric and magnetic forces. This is described in electromagnetic theories.
- Energies Levels: in atoms, beta particles, and electrons have quantized energy levels. Electrons are energy levels, or electron shells that surround the nucleus. Beta particles, if incorporated into an atom via the process of beta decay will also be occupying the quantized levels of energy.
- fundamental particles: Beta particles and electrons are two of the fundamental particles that are the foundation of the common particle physics model that describes the basic particles of matter and their interactions.
- contribution to Nucleosynthesis: In astrophysics and cosmology, beta and electrons participate in a variety of nucleosynthesis processes, which contribute to the formation of elements that are found within stars as well as the beginning of the universe.
- Conservation of Charge: Both beta particles and electrons are subject to the law of the conservation of charge in electric systems, which means the total electric charge within any closed system remains the same. Both beta and electrons have charges that ensure that this conservation law is in place.
Although beta particles and electrons possess distinct properties and their origins They share several characteristics that illustrate the interconnectedness between subatomic particles within the fundamental concepts of particle physics and quantum mechanics.
Applications and Significance
Applications and Significance of Electrons and Beta Particles:
Electron Applications:
- Electronics: Electrons constitute the electronic powerhouse, supplying electricity to devices such as computers televisions, smartphones, and other devices. Understanding the behavior of electrons is essential to designing and developing electronic technology.
- Electricity Generation: Electrons play a role in the production of electricity at power plants. They travel through conductive materials producing electric current and facilitating the transfer of electricity to industrial and residential homes.
- Chemical Reactions: Electrons are the primary factor that determines the chemical properties of elements and play a role during chemical reactions. This information is essential in understanding and controlling chemical reactions, from the simplest chemical synthesis process to industrial processes.
- Spectroscopy: Electrons play an important role in spectroscopic techniques utilized in physics, chemistry, and astronomy, to investigate the interaction between matter and electromagnetic radiation. Techniques such as UV-Vis X-ray and electron spectroscopy are based on the electron’s behavior.
- Materials Science: Understanding electron properties is vital to the study of materials. It is utilized in the creation of new materials for different applications, such as construction, aerospace, and manufacturing.
Beta Particle Applications:
- Radiation Therapy: Beta particles with high energy (electron beams) are employed as radiation therapies to fight cancerous tumors. They attack and destroy cancer cells while minimizing the damage to healthy tissue around them.
- Radiometric Dating: Beta decay has been utilized in radiometric dating methods for determining the age of geological and archaeological samples. For instance, carbon-14 dating is based on its decay in carbon-14 (a beta emitter) in organic substances.
- Nuclear Medicine: Beta emitters are employed to aid in the use of nuclear medicine, both for therapeutic and diagnostic goals. Beta particles from radioactive substances are used in imaging (e.g. Positron Emission Tomography, or PET scans) as well as targeted cancer treatments.
- Particle Detectors: Particles of beta are measured and detected by particle detectors, including Geiger-Muller counters as well as scintillation detectors. These detectors are utilized in a variety of scientific experiments, for instance, those carried out within particle accelerators.
- Nuclear Research: Processes of Beta decay form essential to research in nuclear science which aids scientists in understanding the behavior of nuclear nuclei and assisting in understanding the forces fundamental to subatomic particles.
- Environmental Monitoring: Particle detectors in beta are used for evaluating and monitoring the level of radiation from the environment as well as potential risks. This is essential for ensuring the safety of areas with nuclear facilities as well as for assisting in the event of nuclear accidents.
- Energy generation: Beta decay is utilized in betavoltaic devices to produce electricity. It is also possible to use it for power sources that are remote or space-based.
The significance of electrons as well as beta particles for these fields is immense and extends across areas like energy, healthcare particle physics, energy, and environmental protection. A thorough understanding of these particles is vital to advancing technology, improving medical treatment, and determining the properties of our universe.
Detection and Observation
Detection and Observation of Electrons and Beta Particles:
Electrons:
- Electron Microscopy: Electrons can be observed by electron microscopes, like scanning electron microscopes (SEM) and transmission electron microscopes (TEM). These instruments make use of electron beams to provide high-resolution photographs of objects such as biological specimens and nanoscale materials.
- Cloud chambers: Electrons may be observed and detected inside cloud chambers, which are a type of device that contains an extremely saturated vapour. When charged particles such as electrons travel through, they leave distinct tracks that are visible in the vapor permitting direct observation.
- Scintillation Detectors: Scintillation detectors are typically used to detect electrons in indirect ways. When electrons interact with certain substances (scintillators) they produce lights that flash (scintillations) which can be seen and detected. This method is commonly employed in particle physics research.
- bubble chambers: Much like cloud chambers, the bubble chambers function through the creation of a superheated fluid. When an electron travels through the chamber, it creates tiny bubbles that can be seen and provide information on the trajectory of the particle.
- Energy Spectroscopy: Electrons can be identified and their energy measured with electron spectrometers like electrostatic and magnetic analyzers. These instruments can be utilized for scientific research to examine the properties of electrons which include their energy and speed.
Beta Particles:
- Geiger-Muller Counters: Geiger-Muller counters are typically employed for the observation and detection of beta particles. When the beta particle is in contact with the gas in the detector, it emits electrical impulses that can be recorded and counted, giving details about the existence and strength of beta radiation.
- Scintillation Detectors: Scintillation detectors are employed to detect and observe beta particles. If a beta particle comes into contact with a scintillator it emits flashes of light that can be observed and analysed to determine the nature and presence that beta radiation has.
- Bubble chambers: Beta particles, particularly those with high energy, can be seen in bubble chambers in which they form visible trails of bubbles when they travel through superheated liquid.
- Solid-State Detectors: The detectors that are solid-state, like silicon detectors, are utilized in the search for beta particles. They function by assessing the amount of ionization created by beta particles when they come into contact with the detector.
- Radiation Spectrometers: beta particles once discovered, can be studied by using radiation spectrometers to determine their energy spectrum. This is essential for identifying the specific beta-emitting isotopes as well as the energy level they have.
- Nuclear Emulsions: Beta particles can be identified with photographic emulsions made of silver Halide crystals. If a beta particle comes into contact with the emulsion, it creates trails that can be analyzed and observed with a microscope.
These observation and detection methods are crucial in many applications in science and practice that range from basic studies in the field of particle physics to radiation security and monitoring of environmental conditions. They enable researchers and technicians to study and observe the behavior of beta and electron particles in a variety of environments.
Conclusion
Beta particles and electrons, despite their distinct characteristics, are essential to our understanding of the nature of matter and the universe. Electrons power chemistry and electronics, whereas beta particles have applications in nuclear radiation therapy and research.
Both have similar behavior and their significance in the field of science and technology, which highlights their significance in expanding our understanding and improving practical applications.
