Surfactants play a vital part in different businesses, counting individual care, family cleaning, agribusiness, and pharmaceuticals. They are versatile compounds that help reduce surface tension, enhance wetting, and stabilize emulsions. Surfactants Can Be Broadly Classified Into Three Fundamental Categories Anionic Cationic and Nonionic Surfactants. We are going investigate the key contrasts between these surfactant sorts and their applications.
Introduction
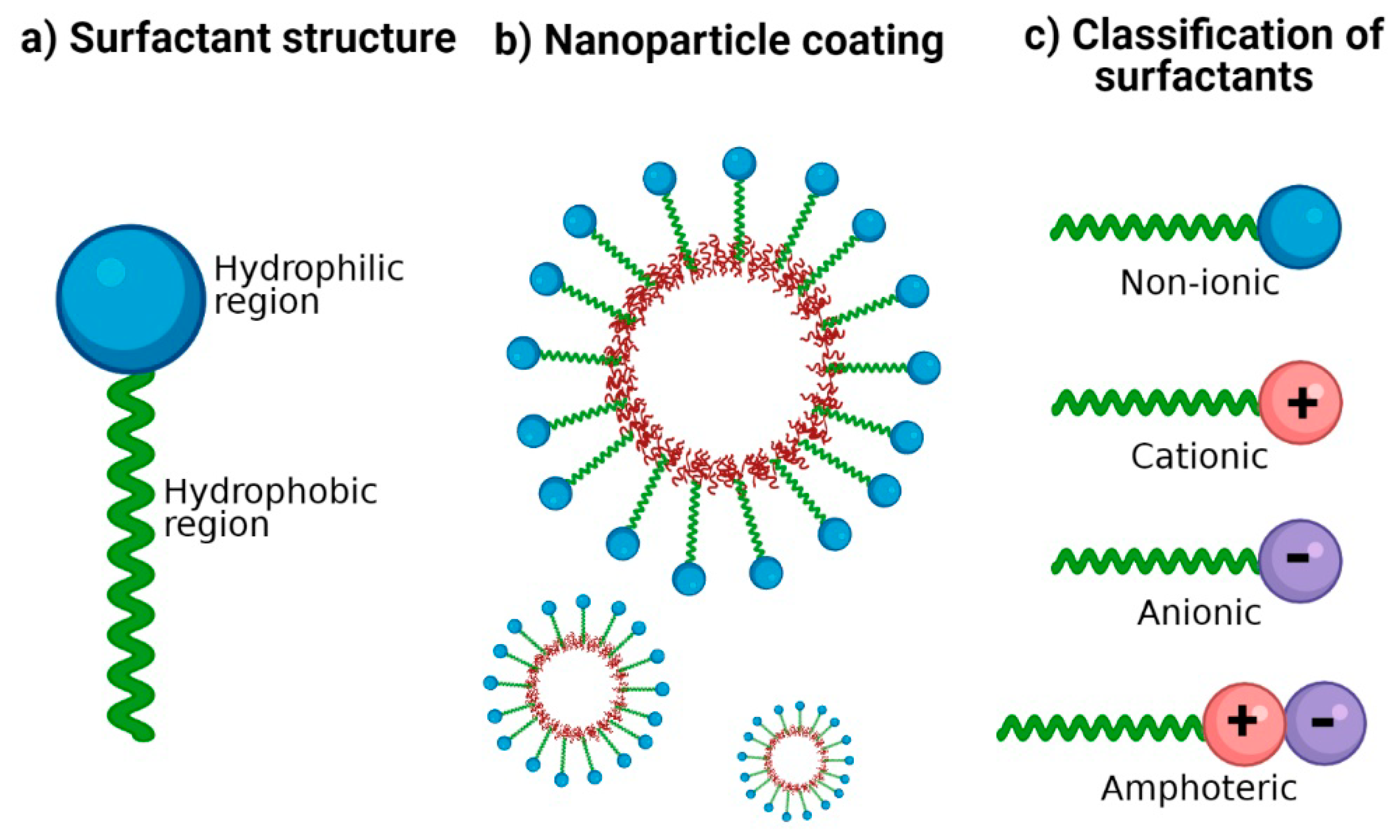
Surfactants, brief for surface-active specialists, are compounds that contain both hydrophobic (water-repellent) and hydrophilic (water-attracting) components. This interesting atomic structure permits them to be connected with both water and oil, making them profoundly viable in a wide run of applications. Surfactants lower the interfacial tension between two immiscible substances, such as oil and water, enabling them to mix and form stable emulsions.
Definition of Anionic Cationic and Nonionic Surfactants
Anionic Surfactants: Anionic surfactants are a sort of surfactant that contains an adversely charged hydrophilic (water-loving) gather. This group is usually derived from carboxylate, sulfate, sulfonate, or phosphate functional groups. The negative charge on the hydrophilic group allows anionic surfactants to be more soluble in water. They are commonly utilized in items like cleansers, cleansers, shampoos, and cleaning specialists due to their capacity to evacuate soil, oil, and oil from surfaces. Illustrations of Anionic Surfactants Incorporate Sodium Lauryl Sulfate (SLS) and Sodium dodecylbenzenesulfonate (SDBS).
Cationic Surfactants: Cationic surfactants are surfactants that contain an emphatically charged hydrophilic bunch. This positive charge is typically provided by quaternary ammonium compounds. Cationic surfactants are frequently utilized as disinfectants, texture conditioners, hair conditioners, and in individual care items. They are effective at binding to negatively charged surfaces and can provide antimicrobial properties. A Few Common Illustrations of Cationic Surfactants Are Cetyltrimethylammonium Bromide (CTAB) and Benzalkonium Chloride.
Nonionic Surfactants: Nonionic Surfactants Are Surfactants That Don’t Carry an Electrical Charge. They have a hydrophilic gather that’s regularly composed of polyethylene oxide (PEO) or polypropylene oxide (PPO) chains. Nonionic Surfactants Are Known for Their Great Steadiness, Compatibility With Other Surfactants and Gentleness on the Skin. They are commonly used in products such as cosmetics, pharmaceuticals, textiles, and emulsion polymerization. A few well-known cases of nonionic surfactants are polyethylene glycol (PEG) and ethoxylated greasy alcohol.
Anionic surfactants have a contrarily charged hydrophilic gather, cationic surfactants have an emphatically charged hydrophilic gather, and nonionic surfactants don’t carry any charge. Each sort of surfactant has one-of-a-kind properties and is utilized in totally different applications based on their chemical characteristics.
Importance of surfactants in various industries
Surfactants play a crucial role in various industries due to their unique properties and capabilities.
Some of the key importance of surfactants in different industries are:
1. Personal Care and Cosmetics: Surfactants are widely used in personal care products such as shampoos, body washes, soaps, and toothpaste. They help in the formation of stable emulsions, enhance foaming and cleansing properties, and improve the spreadability of products. Surfactants also contribute to the solubilization of ingredients, making them more effective in delivering desired benefits.
2. Household Cleaning: Surfactants are essential components in household cleaning products like detergents, dishwashing liquids, and surface cleaners. They lower the surface tension of water, enabling it to wet surfaces more effectively and facilitate the removal of dirt, grease, and stains. Surfactants also aid in the suspension of soils and prevent redeposition, ensuring efficient cleaning.
3. Textile Industry: Surfactants are used in textile processing for tasks such as wetting, scouring, emulsification, and dyeing. They assist in the even distribution of dyes and pigments, improve dyeing efficiency, and enhance the colorfastness of textiles. Surfactants also aid in the removal of impurities and facilitate better penetration of chemicals during textile treatments.
4. Agriculture: Surfactants are utilized in agricultural practices to improve the effectiveness of pesticides, herbicides, and fertilizers. They help in reducing surface tension and allow uniform spreading and coverage of agrochemicals on plant surfaces. Surfactants can enhance the absorption and penetration of active ingredients, leading to better crop protection and nutrient uptake.
5. Pharmaceuticals: Surfactants are utilized in the formulation of pharmaceutical products such as creams, ointments, and drug delivery systems. They assist in solubilizing hydrophobic drugs, improving drug stability, and enhancing the absorption and bioavailability of active pharmaceutical ingredients. Surfactants also contribute to the formation of stable emulsions and microemulsions in drug formulations.
6. Oil and Gas Industry: Surfactants find applications in the oil and gas industry for tasks like oil recovery, drilling, and emulsion breaking. They aid in reducing the interfacial tension between oil and water, facilitating the displacement of oil from reservoir rocks during enhanced oil recovery methods. Surfactants also assist in emulsion control, preventing the formation and stabilizing of unwanted oil-water emulsions.
7. Paints and Coatings: Surfactants are used in paints, coatings, and inks to improve the wetting, dispersion, and stability of pigments. They aid in achieving uniform coverage and adhesion of coatings on surfaces, enhance color development, and prevent agglomeration or settling of particles. Surfactants also contribute to the flow and leveling properties of paints, ensuring smooth application.
Surfactants are indispensable in various industries due to their ability to modify surface properties, enhance solubility, improve dispersion, and facilitate desired interactions. Their versatility and unique functions make them essential components in a wide range of products and processes across industries.
What are Anionic Surfactants?
Anionic surfactants are a course of surfactants that have a contrarily charged hydrophilic (water-loving) gather. The negative charge is typically derived from functional groups such as carboxylate (-COO-), sulfate (-OSO3-), sulfonate (-SO3-), or phosphate (-PO4-) groups. The presence of this charged group imparts anionic surfactants with specific properties and behaviors.
Anionic surfactants are exceedingly dissolvable in water due to their hydrophilic nature. The negatively charged hydrophilic group allows them to interact effectively with water molecules, forming hydrogen bonds and electrostatic attractions. As a result, anionic surfactants exhibit excellent wetting ability and are capable of reducing the surface tension of water.
The hydrophobic (water-repelling) part of anionic surfactants consists of a long hydrocarbon chain. This hydrophobic parcel is capable for their capacity to break down into oils, fats, and other nonpolar solvents. The combination of hydrophilic and hydrophobic components in anionic surfactants leads to the arrangement of micelles in fluid arrangements. Micelles are spherical aggregates where the hydrophilic heads of the surfactant molecules face outward, interacting with the surrounding water, while the hydrophobic tails remain in the interior, shielded from the aqueous environment.
Anionic surfactants are broadly utilized in different businesses and items due to their amazing surfactant properties. They have strong detergent and foaming abilities, making them effective in removing dirt, oil, and grease from surfaces. Anionic surfactants are commonly found in family cleaning items, such as clothing cleansers, dishwashing fluids, and all-purpose cleaners.
Anionic surfactants are utilized in individual care items like shampoos, body washes, and cleansers due to their capacity to make a wealthy foam and give viable cleansing. They offer assistance in emulsifying and stabilizing oil-in-water emulsions, which permits the definition of creams, salves, and other corrective items.
It is important to consider the environmental impact of anionic surfactants. When released into water systems, they can have detrimental effects on aquatic organisms. A few anionic surfactants are known to be harmful and have the potential to endure within the environment. Therefore, there is a need to use and dispose of anionic surfactants responsibly to minimize their impact on ecosystems.
Anionic surfactants are surfactants with a negatively charged hydrophilic group. They possess excellent solubility in water, form micelles, and exhibit strong detergent and foaming properties. They are extensively used in cleaning products, personal care items, and other applications where effective wetting and emulsification are required.
Common examples of anionic surfactants
There are several common examples of anionic surfactants that are widely used in various industries.
Some of These Examples include:
1. Sodium Lauryl Sulfate (SLS): SLS Is One of the Most Commonly Used Anionic Surfactants. It is broadly found in individual care items such as shampoos, toothpaste, and body washes due to its amazing frothing and cleansing properties.
2. Sodium Laureth Sulfate (SLES): SLES is another popular anionic surfactant used in personal care products. It is similar to SLS but undergoes ethoxylation, which makes it milder and less irritating to the skin.
3. Linear Alkylbenzene Sulfonate (LAS): LAS is commonly used in laundry detergents and dishwashing liquids. It has strong cleaning power and excellent compatibility with other ingredients, making it a versatile anionic surfactant.
4. Sodium Cocoyl Isethionate (SCI): SCI is a mild anionic surfactant derived from coconut oil. It is often found in solid or syndet (synthetic detergent) bars, where it provides gentle cleansing and produces a creamy lather.
5. Sodium Lauroyl Sarcosinate: This anionic surfactant is known for its gentle cleansing properties and low irritation potential. It is commonly utilized in individual care items, counting facial cleansers and child shampoos.
6. Alpha Olefin Sulfonates (AOS): AOS is derived from olefin feedstocks and is used in various applications such as household cleaners, industrial detergents, and agricultural formulations. It exhibits good cleaning performance and compatibility with other surfactants.
7. Sodium Dodecyl Sulfate (SDS): SDS, moreover known as sodium lauryl sulfate (SLS), maybe a flexible anionic surfactant utilized in a wide run of applications, counting cleansers, emulsion polymerization, and protein examination in research facilities.
8. Sodium Alkyl Ether Sulfates (AES): AES surfactants are ethoxylated derivatives of fatty alcohols and are commonly used in personal care products. They provide excellent foaming, emulsifying, and cleaning properties.
It is critical to note that these are fair illustrations of commonly utilized anionic surfactants, and there are numerous other anionic surfactants accessible with changing properties and applications.
What are Cationic Surfactants?
Cationic surfactants are a course of surfactants that have an emphatically charged hydrophilic (water-loving) bunch. The positive charge is typically provided by quaternary ammonium compounds. Cationic surfactants have unique properties and are widely used in various industries.
The hydrophilic part of cationic surfactants contains a positively charged nitrogen atom. This positive charge enables cationic surfactants to be attracted to negatively charged surfaces, such as those found in fabrics, hair, and cell membranes. This property allows cationic surfactants to have excellent substantivity and adhesion to surfaces.
The hydrophobic (water-repelling) portion of cationic surfactants consists of a long alkyl chain. This hydrophobic tail provides the surfactant with solubility in oils and other nonpolar solvents.
Due to their positive charge, cationic surfactants exhibit different behaviors and functions compared to anionic or nonionic surfactants.
Some key characteristics and applications of cationic surfactants include:
1. Antimicrobial Properties: Cationic surfactants are successful against a wide run of microorganisms, counting microbes, parasites, and infections. They disrupt the cell membranes of these microorganisms, leading to their inactivation or destruction. Cationic surfactants are utilized in disinfectants, sanitizers, and additives in different businesses, counting healthcare, individual care, and family cleaning.
2. Fabric Softening: Cationic surfactants are commonly used in fabric softeners due to their ability to adsorb onto fabric surfaces, reducing static electricity and enhancing softness. They also improve the rewetting properties of fabrics, making them feel smoother and more comfortable.
3. Hair Conditioning: Cationic surfactants are frequently used in hair conditioners and detanglers. They help to reduce friction, improve wet and dry combing, and provide a soft and smooth feel to the hair. Cationic surfactants can also neutralize the negative charge on the hair surface, reducing static electricity and improving manageability.
4. Flocculation and Coagulation: Cationic surfactants can be used to destabilize colloidal particles in water, promoting their aggregation and settling. This property makes them valuable in water treatment processes, where they aid in the removal of suspended solids and clarification of water.
5. Emulsification: Cationic surfactants can be used as emulsifiers in the formulation of creams, lotions, and ointments. They help to stabilize oil-in-water emulsions by reducing interfacial tension and preventing phase separation.
6. Personal Care Products: Cationic surfactants are used in various personal care products, including hair care, skin care, and oral care products. They contribute to the formulation of effective cleansers, conditioners, antistatic agents, and preservatives.
It is important to note that cationic surfactants may have some limitations and considerations. They can have reduced compatibility with anionic surfactants and may be less effective in high-pH environments. Cationic surfactants are by and large not appropriate for utilization in items that come into contact with mucous films or require broad flushing, as they can cause a disturbance.

Cationic surfactants play a crucial role in numerous applications, leveraging their positive charge and unique properties to provide benefits in areas such as antimicrobial activity, fabric softening, hair conditioning, and water treatment.
Structure and properties of cationic surfactants
Cationic surfactants have an unmistakable structure and have particular properties that make them one of a kind compared to other sorts of surfactants.
Here is a Diagram of the Structure and Properties of Cationic surfactants:
Structure: Cationic Surfactants Regularly Comprise a Hydrophilic Head Bunch, a Hydrophobic Tail, and an Emphatically Charged Nitrogen Molecule. The hydrophilic head group carries the positive charge and is often derived from quaternary ammonium compounds. The hydrophobic tail is typically a long alkyl chain, which provides the surfactant with solubility in nonpolar solvents.
The presence of the positively charged nitrogen atom is a key characteristic of cationic surfactants. It gives them the ability to interact with negatively charged surfaces, such as those found in fabrics, hair, and cell membranes.
Properties:
1. Surface Activity: Cationic surfactants exhibit surface activity, meaning they can lower the surface tension of liquids and reduce the interfacial tension between immiscible phases. They can adsorb onto interfaces, such as the air-liquid or liquid-liquid interface, and form oriented monolayers.
2. Wetting and Spreading: Cationic surfactants have good wetting properties, allowing them to spread easily on surfaces. This property is beneficial in applications where complete coverage and contact with surfaces are desired, such as in fabric softeners or hair conditioners.
3. Surfactant Interaction: Cationic surfactants can interact with other surfactants, including anionic, nonionic, and amphoteric surfactants. However, their compatibility may be limited with anionic surfactants due to the opposite charges. Cationic surfactants tend to create insoluble complexes or accelerate when blended with anionic surfactants.
4. Antimicrobial Activity: Cationic surfactants are known for their antimicrobial properties. The positively charged head group allows them to disrupt and destabilize microbial cell membranes, leading to the inactivation or destruction of microorganisms such as bacteria, fungi, and viruses.
5. Substantivity and Adhesion: Cationic surfactants exhibit substantivity, meaning they have an affinity to adhere to surfaces. This property allows them to provide longer-lasting effects when applied to fabrics, hair, or skin. The positive charge of cationic surfactants enables them to bind to negatively charged surfaces, enhancing their adhesion.
6. Compatibility: Cationic surfactants are generally compatible with nonionic and amphoteric surfactants. They can form mixed micelles and synergistic combinations that enhance the performance of formulations.
7. pH Sensitivity: The properties and performance of cationic surfactants can be influenced by pH. They are most effective in acidic to neutral pH ranges. At higher pH levels, the positive charge on the nitrogen atom can be partially neutralized, reducing their efficacy.
8. Environmental Considerations: Cationic surfactants can have a higher potential for toxicity and environmental persistence compared to other types of surfactants. It is important to use cationic surfactants responsibly and consider their impact on ecosystems.
These properties make cationic surfactants well-suited for applications such as fabric softeners, hair conditioners, disinfectants, and water treatment processes where their positive charge and unique characteristics provide specific benefits.
What are Nonionic Surfactants?
Nonionic surfactants are a course of surfactants that don’t have an ionic charge in their hydrophilic (water-loving) head bunch. Not at all like anionic surfactants (which have an adversely charged head gather) or cationic surfactants (which have an emphatically charged head bunch), nonionic surfactants are electrically unbiased. They are widely used in various industries and have unique properties and applications.
The hydrophilic part of nonionic surfactants consists of polar functional groups such as hydroxyl (-OH), ethylene oxide (-OCH2CH2O-), or amide (-CONH2) groups. These functional groups do not carry a net charge and are relatively unreactive. This lack of charge makes nonionic surfactants less sensitive to pH and ionic strength compared to anionic or cationic surfactants.
The hydrophobic (water-repelling) parcel of nonionic surfactants ordinarily comprises a long alkyl chain or a combination of alkyl chains. This hydrophobic tail provides the surfactant with solubility in nonpolar solvents such as oils and fats.
Nonionic surfactants exhibit several key properties and characteristics, including:
1. Low Foaming: Nonionic surfactants generally produce low levels of foam compared to anionic or cationic surfactants. This property is beneficial in applications where foam formation needs to be minimized, such as in certain industrial processes or specific cleaning applications.
2. Excellent Detergency: Nonionic surfactants have good detergency and are effective at removing various types of dirt, oils, and greases from surfaces. They can enter and solubilize hydrophobic substances, making them reasonable for utilization in clothing cleansers, dishwashing fluids, and mechanical cleaning items.
3. Good Emulsification: Nonionic surfactants are capable of forming and stabilizing oil-in-water and water-in-oil emulsions. They can help disperse and solubilize immiscible substances, such as oils in water or water in oils. Nonionic surfactants are commonly utilized within the definition of creams, lotions, and corrective items to realize steady emulsions.
4. Mildness and Low Irritation: Nonionic surfactants are generally considered mild and less likely to irritate compared to anionic or cationic surfactants. Their unbiased nature and general moo reactivity make them appropriate for utilization in individual care items, such as shampoos, body washes, and facial cleansers, where gentleness is critical.
5. Compatibility: Nonionic surfactants are compatible with various types of surfactants, including anionic, cationic, and other nonionic surfactants. They can be utilized in combination with other surfactants to improve execution and make custom-fitted definitions.
6. Temperature Stability: Nonionic surfactants generally exhibit good stability and performance over a wide range of temperatures. They Can Maintain Their Effectiveness and Properties Under Both High and Low-Temperature Conditions, Making Them Versatile for Different Applications.
Nonionic surfactants find applications in numerous industries, including personal care, household cleaning, agriculture, paints and coatings, and industrial processes. Their neutral charge, good detergency, emulsifying capabilities, and mildness make them highly versatile and suitable for a wide range of formulations and products.
Difference Between Anionic Cationic and Nonionic Surfactants
The main differences between Anionic Cationic and Nonionic Surfactants lie in their charged or uncharged nature, their behavior in different pH ranges, and their applications.
Here’s a breakdown of the key differences:
1. Charge:
• Anionic Surfactants: Anionic surfactants carry a negatively charged hydrophilic head group. This negative charge enables them to interact with positively charged particles or surfaces.
• Cationic Surfactants: Cationic surfactants have a positively charged hydrophilic head group. This positive charge allows them to interact with negatively charged particles or surfaces.
• Nonionic Surfactants: Nonionic surfactants do not have a charged hydrophilic head group. They are electrically impartial, which implies they have no partiality for charged particles or surfaces.
2. pH Sensitivity:
• Anionic Surfactants: Anionic surfactants are most effective in alkaline or neutral pH conditions. In acidic environments, their negative charge can be neutralized, affecting their performance.
• Cationic Surfactants: Cationic surfactants are most effective in acidic or neutral pH conditions. In alkaline environments, their positive charge can be neutralized, impacting their performance.
• Nonionic Surfactants: Nonionic surfactants are generally pH-independent and can maintain their performance across a wide pH range, from acidic to alkaline conditions.
3. Applications:
• Anionic Surfactants: Anionic surfactants are commonly used in applications requiring foaming, emulsification, and cleaning properties. They are found in items such as shampoos, cleansers, and family cleaners.
• Cationic Surfactants: Cationic surfactants are used for their antimicrobial properties, fabric softening, and hair conditioning. They are commonly found in texture conditioners, hair conditioners, and disinfectants.
• Nonionic Surfactants: Nonionic surfactants are versatile and used in a variety of applications. They are often employed in products where low foaming, emulsification, and mildness are desired, such as in cosmetics, pharmaceuticals, and industrial formulations.
4. Compatibility:
• Anionic Surfactants: Anionic surfactants are generally incompatible with cationic surfactants due to their opposite charges. They can form insoluble complexes or precipitates when mixed.
• Cationic Surfactants: Cationic surfactants are generally incompatible with anionic surfactants due to their opposite charges. Blending them can lead to the arrangement of insoluble complexes or accelerates.
• Nonionic Surfactants: Nonionic surfactants are compatible with anionic, cationic, and other nonionic surfactants. They can be easily combined with other surfactants to achieve desired formulation properties.
It is imperative to note that these contrasts are common characteristics, and there are different sorts and varieties of Anionic Cationic and Nonionic Surfactants, each with their claim particular properties and applications.
Comparison Chart
Here’s a comparison chart highlighting the key differences between Anionic Cationic and Nonionic Surfactants:
| Property | Anionic Surfactants | Cationic Surfactants | Nonionic Surfactants |
|---|---|---|---|
| Charge on Head Group | Negative | Positive | Neutral |
| pH Sensitivity | Effective in alkaline or neutral pH | Effective in acidic or neutral pH | pH independent |
| Interactions | Attracts positively charged particles or surfaces | Attracts negatively charged particles or surfaces | No charge-based interactions |
| Foaming Properties | High foaming | Moderate foaming | Low foaming |
| Applications | Detergents, cleaners, foaming agents | Fabric softeners, hair conditioners, disinfectants | Cosmetics, pharmaceuticals, mild formulations |
| Compatibility | Incompatible with cationic surfactants | Incompatible with anionic surfactants | Compatible with other surfactant types |
| Examples | Sodium lauryl sulfate, sodium dodecylbenzenesulfonate | Benzalkonium chloride, cetyltrimethylammonium bromide | Polysorbate 20, polyethylene glycol |
Please note that this is a general comparison and not an exhaustive list. The specific properties and applications of surfactants can vary depending on their chemical structure and formulation.
Conclusion
Anionic Cationic and Nonionic Surfactants are essential components in various industries. Each type offers distinct properties and advantages. Anionic surfactants are effective cleaners but can be sensitive to water hardness.
Cationic surfactants provide antimicrobial properties but have limited compatibility with anionic ingredients. Nonionic surfactants are mild and versatile but may have lower surface activity. Choosing the right surfactant requires considering the specific requirements of the application and balancing performance, compatibility, and environmental impact.