Cyclins and Cyclin-Dependent Kinases What Are Their Purpose and Advantage
Cyclins and CDKs (cyclin-dependent kinases) play an integral part in orchestrating cell division and replication cycles – essential processes which promote proper growth, division, and replication processes within cells.
Cyclins are a family of proteins that exhibit periodic expression and play a crucial role in driving the cell cycle forward. They are named cyclins because their levels oscillate cyclically throughout the cell cycle.
Cyclins bind to CDKs to activate them and form cyclin-CDK complexes, which then phosphorylate specific target proteins to orchestrate the progression of the cell cycle.
CDKs, conversely, are a group of enzymes that are inactive or partially active in their unbound state. They become active only when they bind to specific cyclin partners.
CDKs act as key cell cycle regulators by phosphorylating target proteins involved in various cell cycles events, such as DNA replication, chromosome segregation, and cell division.
Cyclins are proteins that undergo periodic expression and bind to CDKs, whereas CDKs are enzymes that become active upon binding to cyclins. Together, cyclins and CDKs form cyclin-CDK complexes, which are essential for driving the cell cycle progression.
Importance of cyclins and CDKs in cell cycle regulation
Cyclins and CDKs play an indispensable part in controlling cell cycle progression. Here are some key points highlighting their importance:
- Timing and Coordination: Cyclins and CDKs regulate the orderly progression of the cell cycle by ensuring that each phase occurs at the right time and in the correct sequence. Synchronizing key events within cells such as DNA replication, chromosome segregation, and cell division to maintain genomic integrity and avoid abnormal cell division is vitally important to maintaining genomic integrity and avoiding aberrant cell division.
- Cell Cycle Checkpoints: Cyclins and CDKs contribute to the activation of cell cycle checkpoints, which are surveillance mechanisms that monitor the integrity of DNA and cellular structures at specific points in the cell cycle. These checkpoints halt cell cycle progression if any abnormalities or damage are detected, allowing time for DNA repair or initiation of programmed cell death (apoptosis) if necessary.
- Regulation of Cyclin-CDK Complex Activity: Cyclins and CDKs control the activity of cyclin-CDK complexes through phosphorylation and dephosphorylation events. This regulation ensures that the complexes are active at the appropriate times and locations during the cell cycle. Precise temporal and spatial control of cyclin-CDK activity is crucial for the accurate execution of cell cycle events.
- Substrate Specificity: Cyclin-CDK complexes selectively phosphorylate specific target proteins involved in cell cycle progression. Phosphorylation by cyclin-CDK complexes can activate or inactivate target proteins, leading to the activation or suppression of key cell cycle regulatory pathways. This substrate specificity allows cyclins and CDKs to exert precise control over various cellular processes during the cell cycle.
- Dysregulation and Disease: Aberrant expression or activity of cyclins and CDKs is associated with various diseases, including cancer. Alterations in the levels or function of cyclins and CDKs can disrupt the delicate balance of cell cycle regulation, leading to uncontrolled cell growth, genomic instability, and tumor development. Understanding the dysregulation of cyclins and CDKs in disease can provide insights into the development of targeted therapies for conditions such as cancer.
Cyclins and CDKs are essential components of cell cycle regulation, ensuring proper timing, coordination, and fidelity of cell division. Their precise control over the cell cycle is crucial for maintaining genomic stability and preventing disease.
Cyclins
Cyclins are proteins that play an essential part in controlling cell cycle progression. They are named cyclins because their levels undergo cyclical changes throughout the cell cycle, with their abundance rising and falling at specific stages.
Key aspects of cyclins include:
- Function: Cyclins function as regulatory subunits that bind to cyclin-dependent kinases (CDKs) to form active cyclin-CDK complexes. These complexes phosphorylate target proteins, thereby driving the cell cycle forward and regulating various cell cycles events, such as DNA replication, chromosome segregation, and cell division.
- Types of Cyclins: Different cyclins are expressed and active at different stages of the cell cycle, ensuring proper coordination of cellular processes.Some major types of cyclins include:
- G1 Cyclins: These cyclins are involved in regulating the transition from the G1 phase to the S phase of the cell cycle. They help initiate DNA replication and prepare the cell for entry into the S phase.
- S Cyclins: S cyclins are essential for DNA replication. They promote the assembly and activation of the replication machinery, ensuring accurate duplication of the genetic material.
- G2 Cyclins: G2 cyclins control the transition from the S phase to the G2 phase, regulating the preparation of the cell for mitosis. They help ensure that DNA replication is complete and the cell is ready for division.
- M Cyclins: M cyclins are crucial for the progression of the cell cycle into mitosis. They promote the assembly and activation of the mitotic spindle, which facilitates proper chromosome segregation and cell division.
- Regulation of Cyclin Expression: The expression of cyclins is tightly regulated to ensure their timely presence during specific phases of the cell cycle. Regulation occurs at both transcriptional and post-translational levels. Transcription factors and cell cycle regulators regulate the production of cyclin mRNA while various signaling pathways and posttranslational modifications (phosphorylation or ubiquitination) regulate its stability or degradation.
- Role in Cell Cycle Checkpoints: Cyclins also play a role in the activation of cell cycle checkpoints, which are critical surveillance mechanisms that monitor the integrity of DNA and cellular structures. By cooperating with CDKs, cyclins contribute to the proper functioning of these checkpoints, ensuring accurate cell cycle progression and preventing the propagation of damaged DNA.
Cyclins are regulatory proteins that oscillate in abundance throughout the cell cycle and work in conjunction with CDKs to control cell cycle progression. Their expression and activity are tightly regulated, allowing for precise temporal coordination of cell cycle events.
Cyclin-Dependent Kinases (CDKs)
CDKs are enzymes that play a vital role in the control of cell cycle progression. C-dependent proteins have earned this title due to the fact that their activity relies upon binding with specific cyclin proteins.
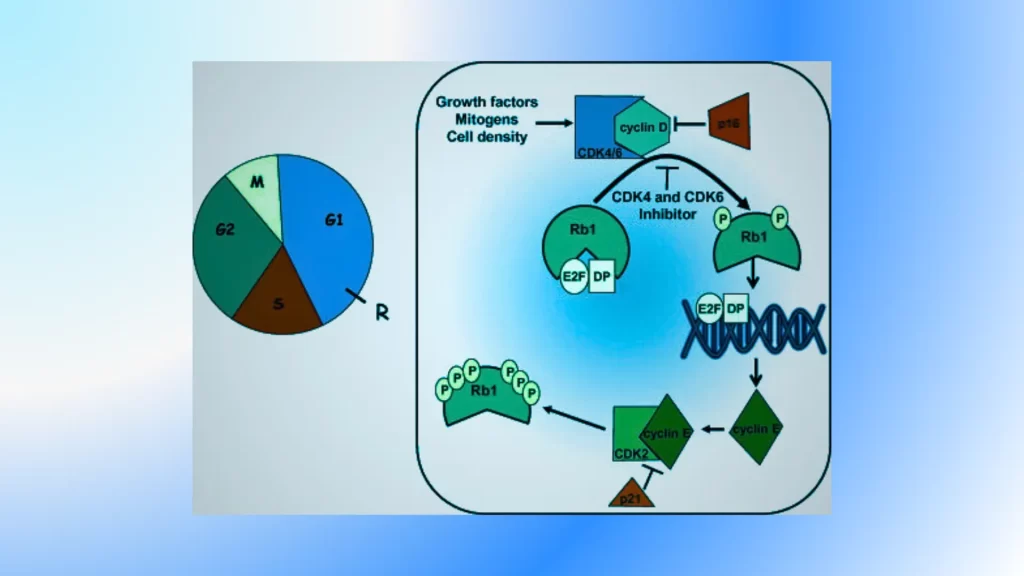
CDKs work in partnership with cyclins to control the progression of the cell cycle and ensure proper coordination of cellular processes.
Here are some key aspects of CDKs:
- Function: CDKs, or serine/threonine kinases, catalyze the transfer of phosphate groups from ATP onto target proteins’ serine or threonine residues via catalysis. CDKs phosphorylate a wide range of target proteins involved in cell cycle regulation, thereby modulating their activity and function. CDKs play a central role in driving the cell cycle forward by controlling transitions between different phases and ensuring the orderly progression of events.
- Activation: CDKs are present in cells in an inactive or partially active state. Their activation requires the binding of specific cyclin proteins, which induce conformational changes and expose the active site of the kinase. The binding of cyclins to CDKs triggers a cascade of events, leading to the full activation of the CDK.
- Substrate Specificity: CDKs phosphorylate specific target proteins involved in cell cycle regulation. These targets include proteins involved in DNA replication, chromosome condensation, spindle assembly, and cell division. The phosphorylation of these targets by CDKs can result in their activation or inactivation, leading to the appropriate progression of the cell cycle.
- Inhibition and Negative Regulation: CDK activity is tightly regulated to ensure proper cell cycle control. In addition to cyclin binding, CDKs can be negatively regulated by inhibitory proteins called CDK inhibitors (CKIs). CKIs bind to cyclin-CDK complexes and prevent their activation or interfere with substrate binding. CKIs play a crucial role in regulating the timing and duration of CDK activity and contribute to cell cycle arrest at specific checkpoints.
- Cell Cycle Checkpoints: CDKs are intimately involved in the activation and regulation of cell cycle checkpoints. Checkpoints are molecular surveillance mechanisms that monitor the fidelity of DNA replication, DNA damage, and chromosome alignment. CDKs, in coordination with cyclins, modulate the activity of crucial checkpoint proteins, allowing cells to pause the cell cycle, repair DNA damage, or initiate programmed cell death (apoptosis) if necessary.
- Dysregulation and Disease: Aberrant activation or inactivation of CDKs can lead to cell cycle dysregulation and contribute to the development of various diseases, including cancer. Alterations in the levels of cyclins, CDKs, or their inhibitors can disrupt the delicate balance of cell cycle control, resulting in uncontrolled cell growth, genomic instability, and tumor formation.
CDKs are enzymes that partner with cyclins to regulate the progression of the cell cycle. Their activity is tightly regulated, and they phosphorylate specific target proteins to drive cell cycle events.
CDKs play an indispensable role in maintaining healthy cell cycle control; therefore, any dysregulation could have serious ramifications for both homeostasis and disease development in cells.
What is the Difference Between Cyclins and Cyclin-Dependent Kinases?
Cyclins and CDKs play key roles in cell cycle regulation. Each has unique responsibilities.
Here are the key differences between cyclins and CDKs:
- Composition: Cyclins and CDKs belong to different protein families; thus their components differ considerably in composition.
- Function: Cyclins act as regulatory subunits that bind to CDKs, forming cyclin-CDK complexes. CDKs, on the other hand, are catalytic subunits that phosphorylate target proteins.
- Activation: Cyclins are responsible for activating CDKs. When cyclins bind to CDKs, it induces conformational changes in CDKs, leading to their activation and kinase activity.
- Activity Regulation: The activity of cyclin-CDK complexes is regulated by the binding and expression of cyclins. CDK activity can also be modulated via posttranslational modifications and inhibitors (CKIs).
- Timing: Cyclins exhibit periodic expression during specific phases of the cell cycle, while CDKs are present throughout the cell cycle. The levels of cyclins rise and fall cyclically to ensure proper coordination of cell cycle events.
- Substrate Specificity: Cyclin-CDK complexes phosphorylate specific target proteins involved in cell cycle regulation, while CDKs alone have limited substrate specificity. The cyclin component of the complex determines the specificity of the phosphorylation events.
- Regulation of Checkpoints: Cyclin-CDK complexes play a role in the activation and regulation of cell cycle checkpoints. They modulate the activity of checkpoint proteins to ensure accurate cell cycle progression. CDKs alone do not directly regulate checkpoints.
- Dysregulation and Disease: Dysregulation of cyclins and CDKs can have different consequences. Dysregulated cyclin expression can lead to uncontrolled cell proliferation and the development of cancer. Dysregulated CDK activity can disrupt the orderly progression of the cell cycle and contribute to genomic instability and disease.
Cyclins and CDKs are distinct components involved in cell cycle regulation. Cyclins act as regulatory subunits, while CDKs are enzymes. Cyclins activate CDKs, and together they form cyclin-CDK complexes that phosphorylate target proteins.
Cyclins exhibit periodic expression, while CDKs are present throughout the cell cycle. Cyclins determine the substrate specificity of the complexes, and dysregulation of cyclins and CDKs can have different implications for cellular processes and disease development.
What is the relationship Between Cyclins and CDKs
Cyclins and CDKs play a pivotal role in cell cycle regulation. Here are the key aspects of their relationship:
- Formation of Cyclin-CDK Complexes: Cyclins and CDKs bind together to form cyclin-CDK complexes. Cyclins act as regulatory subunits and CDKs as catalytic subunits. CDK binding causes conformational changes within CDKs that result in their activation and activate their kinase activity, leading to their activation.
- Activation of CDKs: Cyclin binding is necessary for the full activation of CDKs. Cyclins provide a regulatory function by promoting the proper folding and activation of CDKs, enabling them to phosphorylate target proteins involved in cell cycle regulation.
- Temporal Regulation: Different cyclins are expressed and active at specific stages of the cell cycle. The expression and degradation of cyclins are tightly regulated, ensuring that the corresponding cyclin-CDK complexes are present and active at the appropriate times. This temporal regulation allows for precise coordination of cell cycle events and ensures that they occur in the correct order.
- Substrate Specificity: Cyclin-CDK complexes have specific substrate specificity. The cyclin component of the complex determines the substrate specificity, as different cyclins can associate with the same CDK but phosphorylate other target proteins. The specific cyclin-CDK complexes phosphorylate distinct sets of target proteins, thereby regulating specific processes in the cell cycle.
- Regulation of Cell Cycle Checkpoints: Cyclin-CDK complexes are involved in the activation and regulation of cell cycle checkpoints, which are surveillance mechanisms that ensure the fidelity of DNA replication, repair, and chromosome segregation. Cyclin-CDK complexes modulate the activity of checkpoint proteins, allowing the cell to pause the cell cycle for repair or initiate programmed cell death (apoptosis) if necessary.
- Negative Regulation: The activity of cyclin-CDK complexes can be negatively regulated by CDK inhibitors (CKIs). CKIs are proteins that bind to cyclin-CDK complexes and inhibit their activity. CKIs play a crucial role in regulating the timing and duration of cyclin-CDK activity, allowing for proper control and coordination of cell cycle progression.
Cyclins and CDKs form cyclin-CDK complexes, with cyclins providing regulatory functions and CDKs providing catalytic functions. The formation of these complexes and their activation is crucial for the precise regulation of the cell cycle.
Cyclin-CDK complexes exhibit temporal specificity, and substrate specificity, and contribute to the activation and regulation of cell cycle checkpoints.
Cyclins vs Cyclin-Dependent Kinases in Tabular Form
Sure! Here’s a tabular comparison of cyclins and cyclin-dependent kinases (CDKs):
| Cyclins | Cyclin-Dependent Kinases (CDKs) |
| Proteins | Enzymes |
| Act as regulatory subunits | Act as catalytic subunits |
| Bind to CDKs to form complexes | Bind to cyclins to form complexes |
| Induce conformational changes in CDKs | Are activated by cyclin binding |
| Temporal regulation | Present throughout the cell cycle |
| Periodic expression during specific stages of the cell cycle | Constant presence |
| Determine substrate specificity of cyclin-CDK complexes | Have limited substrate specificity |
| Regulate cell cycle checkpoints | Contribute to checkpoint regulation |
| Dysregulation can lead to uncontrolled cell proliferation and cancer | Dysregulation can disrupt cell cycle progression and contribute to disease |
Please note that while this table highlights the main differences between cyclins and CDKs, it is important to understand that they work in close coordination with cyclin-CDK complexes to regulate the cell cycle.
Examples of Cyclin-CDK Complexes and Their Functions
There are several examples of cyclin-CDK complexes that play important roles in cell cycle regulation.
Here are a few examples along with their functions:
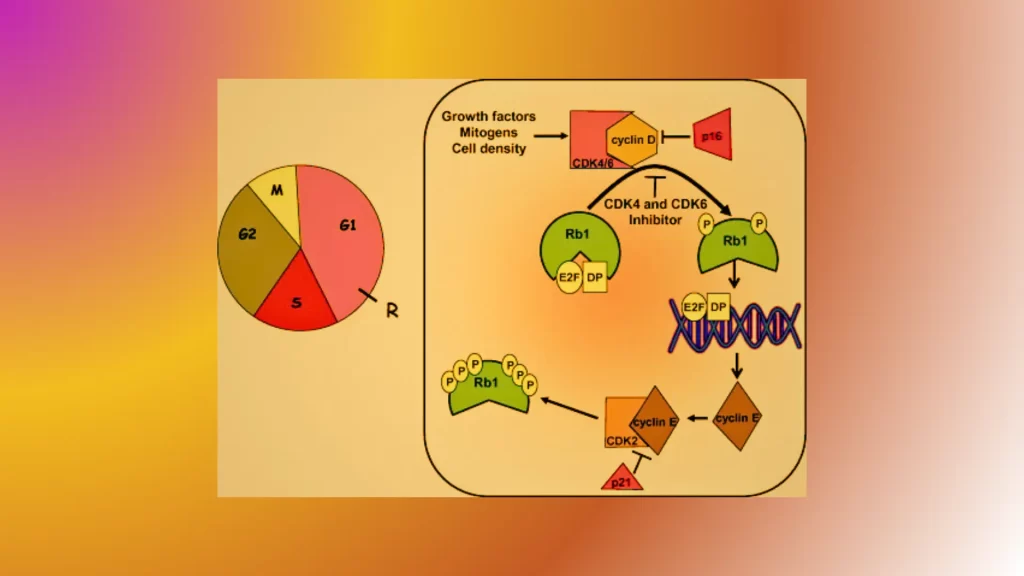
- Cyclin D-CDK4/6 Complex:
-
- Function: Promotes the transition from the G1 phase to the S phase of the cell cycle.
- Role: Phosphorylates and inactivates the retinoblastoma protein (Rb), releasing E2F transcription factors and allowing progression into the S phase.
- Cyclin E-CDK2 Complex:
-
- Function: Controls the G1/S transition.
- Role: Phosphorylates additional Rb proteins and other targets to further promote cell cycle progression into the S phase.
- Cyclin A-CDK2 Complex:
-
- Function: Regulates DNA replication and the transition from S phase to G2 phase.
- Role: Phosphorylates proteins involved in DNA replication and chromatin remodeling, ensuring accurate DNA synthesis and preparation for mitosis.
- Cyclin B-CDK1 Complex (also known as a mitosis-promoting factor or MPF):
-
- Function: Controls the progression from the G2 phase to mitosis.
- Role: Triggers the entry into mitosis by phosphorylating various proteins involved in chromosome condensation, spindle assembly, and nuclear envelope breakdown.
- Cyclin D-CDK4/6 and Cyclin E-CDK2 Complexes:
-
- Function: Promote cell cycle progression in response to growth factor signaling.
- Role: Transmit signals from external cues, such as growth factors, to the cell cycle machinery, allowing cells to proliferate in response to appropriate growth signals.
These are just a few examples of cyclin-CDK complexes and their functions. Notably, CDKs and cyclins work in unison at each step in the cell cycle; different cyclin-CDK complexes serve specific roles at various points throughout.
The phosphorylation events mediated by these complexes regulate various cellular processes, ensuring accurate progression through the cell cycle.
Dysregulation of Cyclins and CDKs in Disease
The dysregulation of cyclins and CDKs may play a vital role in cancer and many other illnesses.
Here are some examples of how dysregulation of cyclins and CDKs contributes to disease:
Cancer:
- Overexpression of cyclins: As with many types of cancer, elevated expression levels for specific cyclins such as D1, E and B1 cyclins is commonly seen. Elevated cyclin levels can lead to abnormal activation of CDKs and uncontrolled cell cycle progression, promoting tumor growth.
- CDK Overactivation: Mutations or amplifications in CDK genes like CDK4 and CDK6 may lead to constitutive activation of CDKs and lead to their constitutive activation. Hyperactive CDKs can bypass cell cycle checkpoints, allowing cells to proliferate even in the presence of DNA damage or other abnormalities.
- Loss of CDK inhibitors (CKIs): Inactivation or reduced expression of CKIs, such as p16INK4A and p27Kip1, can impair the ability to inhibit cyclin-CDK complexes, leading to uncontrolled CDK activity and cell cycle dysregulation.
- Genomic instability: Dysregulated cyclins and CDKs can contribute to genomic instability by promoting aberrant DNA replication, chromosome segregation errors, and impaired DNA repair mechanisms.
Neurodegenerative diseases:
- CDK hyperactivity: Neurodegenerative diseases like Alzheimer’s, Parkinson’s and Huntington’s can result in abnormal activation of CDKs. CDKs may phosphorylate and disrupt neuronal proteins responsible for maintaining neuronal structure and function, contributing to neurodegeneration.
- Tau Phosphorylation CDK5: a CDK-esterified family member most commonly expressed in the brain plays an important role in phosphorylating tau protein. Hyperphosphorylated Tau can destabilize the microtubules in neurons, and cause neurofibrillary Tangles to form – an indication of many neurodegenerative disorders.
Cardiovascular diseases:
- Vascular smooth muscle cell proliferation: Dysregulated cyclin-CDK complexes can contribute to the excessive proliferation of vascular smooth muscle cells, a key event in the development of atherosclerosis and restenosis.
- Cardiac Hypertrophy: Abnormal activation of cyclin-CDK complexes in the heart can result in cardiac hypertrophy, an increase in cardiomyocyte size characterized by abnormal activation. Disrupted cyclin-CDK regulation can disrupt the balance between cell growth and cell division, leading to pathological cardiac hypertrophy.
These are just a few examples of how dysregulation of cyclins and CDKs can contribute to disease. Understanding the aberrant regulation of cyclins and CDKs provides insights into the underlying mechanisms of various disorders and may offer potential targets for therapeutic intervention.
Conclusion
Cyclins and CDKs (cyclin-dependent kinases) play a pivotal role in controlling cell cycle progression. Cyclins act as regulatory subunits, while CDKs serve as catalytic subunits. Their partnership in the form of cyclin-CDK complexes allows for precise control and coordination of cell cycle events.
Cyclins and CDKs work together to ensure the orderly progression of the cell cycle by phosphorylating specific target proteins involved in DNA replication, chromosome segregation, and cell division.
The activation of cyclin-CDK complexes is tightly regulated, with cyclin expression being temporally regulated and CDK activity is modulated by post-translational modifications and CDK inhibitors (CKIs).
