Transition metal complexes play a crucial role. These complexes are composed of a central metal ion surrounded by a group of ligands. The interaction between the metal ion and ligands gives rise to various properties and characteristics, which can be further classified into high spin and low spin complexes. Understanding the difference between high spin and low spin complexes is essential to comprehend their unique behaviors and applications. We will explore these two types of complexes in detail, shedding light on their distinctive features, electron configurations, and magnetic properties.
Definition of High Spin and Low Spin Complexes
High Spin Complexes: High spin complexes refer to coordination complexes in which the electrons occupy the available orbitals in a way that maximizes the number of unpaired electrons. The electrons are distributed across the available d orbitals before any pairing occurs. As a result, high spin complexes tend to have a larger number of unpaired electrons, leading to higher magnetic moments.
Low Spin Complexes: Low spin complexes, on the other hand, are coordination complexes in which the electrons occupy the available orbitals in a way that minimizes the number of unpaired electrons. In these complexes, the electrons are paired up in the d orbitals before filling up the higher energy levels. Consequently, low spin complexes have a smaller number of unpaired electrons, resulting in lower magnetic moments compared to high spin complexes.
The spin state of a complex is determined by several factors, including the strength of the ligand field, the oxidation state of the metal ion, and the coordination number of the complex. These factors influence the energy difference between the d orbitals, which, in turn, affects the distribution of electrons and determines whether a complex will exhibit a high spin or low spin configuration.
Importance of understanding spin states in coordination complexes
Understanding spin states in coordination complexes are crucial for several reasons:
1. Magnetic Properties: The spin state of a coordination complex directly affects its magnetic properties. High spin complexes, with their larger number of unpaired electrons, tend to exhibit stronger magnetic moments compared to low spin complexes. By understanding the spin state, researchers can predict and explain the magnetic behavior of coordination complexes, which is essential for applications such as magnetic data storage, spintronics, and magnetic resonance imaging (MRI).
2. Optical Properties: The spin state of a coordination complex can also influence its color and absorption spectra. Unpaired electrons in high spin complexes can undergo electronic transitions that result in characteristic colors and absorption bands. By contrast, low spin complexes with fewer unpaired electrons may exhibit different colors and absorption spectra. Knowledge of the spin state helps in interpreting and predicting the optical properties of coordination complexes, which is important for fields such as materials science, optics, and dye chemistry.
3. Stability and Reactivity: The spin state of a coordination complex can affect its stability and reactivity. High spin complexes often have weaker ligand-metal bonding due to the presence of unpaired electrons. This can make them more susceptible to ligand substitution reactions and influence their redox properties. In contrast, low spin complexes with stronger ligand-metal bonding may exhibit different reactivity patterns. Understanding the spin state is crucial for predicting the stability and reactivity of coordination complexes, which has implications in catalysis, bioinorganic chemistry, and materials synthesis.
4. Electronic Structure and Bonding: The spin state of a coordination complex is intimately linked to its electronic structure and bonding. High spin and low spin complexes have different orbital filling patterns, leading to distinct energy levels and electronic configurations. This affects the nature and strength of the metal-ligand bonds and can influence the overall stability and properties of the complex. Understanding the spin state provides insights into the electronic structure and bonding interactions within coordination complexes, aiding in the design and understanding of new functional materials and catalysts.
Understanding the spin states of coordination complexes is essential for comprehending and predicting their magnetic, optical, stability, reactivity, and electronic properties. This knowledge has wide-ranging implications in various scientific and technological fields, enabling the rational design and utilization of coordination complexes for diverse applications.
What are High Spin Complexes?
High spin complexes are coordination complexes in which the electrons occupy the available d orbitals in a way that maximizes the number of unpaired electrons. These complexes are characterized by a higher number of unpaired electrons and a larger magnetic moment compared to low spin complexes.
The electrons are distributed across the available d orbitals before any pairing occurs. This means that electrons occupy different orbitals with parallel spins, resulting in the maximum number of unpaired electrons. The distribution of electrons in high spin complexes follows Hund’s rule, which states that electrons will occupy separate orbitals with the same spin before pairing occurs.
The spin state of a coordination complex is influenced by several factors, including the strength of the ligand field, the oxidation state of the central metal ion, and the coordination number of the complex. High spin configurations are favored when the ligand field is weak, the metal ion has a higher oxidation state, and/or the coordination number is high.
The presence of unpaired electrons in high spin complexes has implications for their magnetic properties. High spin complexes exhibit stronger magnetic moments due to the presence of multiple unpaired electrons, which can interact with external magnetic fields. This property makes high spin complexes useful in various applications such as magnetic data storage, spintronics, and magnetic resonance imaging (MRI).
Examples of high spin complexes include octahedral complexes such as [Fe(H2O)6]2+ and tetrahedral complexes such as [NiCl4]2-. These complexes exhibit a high spin configuration due to the weak ligand field or the presence of a high coordination number.
![Why is [math][Cr (NH_3)] ^ {3+}[/math] a high spin complex? - Quora](https://qph.cf2.quoracdn.net/main-qimg-39c6ab9fa9f76d4aeb65987cf0c19533.webp)
High spin complexes are coordination complexes with a larger number of unpaired electrons, resulting in stronger magnetic moments and distinct properties compared to low spin complexes.
Factors influencing the high spin configuration
Several factors influence the high spin configuration in coordination complexes. These factors determine the extent to which electrons occupy the available d orbitals with parallel spins, resulting in a higher number of unpaired electrons.
The key factors influencing the high spin configuration are:
1. Ligand Field Strength: The ligands surrounding the central metal ion in a coordination complex create a ligand field, which influences the energy levels of the d orbitals. Strong ligand fields, such as small and highly charged ligands, cause a larger energy difference between the d orbitals. In such cases, the energy cost of pairing electrons becomes significant, favoring the high spin configuration with maximum unpaired electrons.
2. Metal Ion Oxidation State: The oxidation state of the central metal ion affects the energy levels of the d orbitals. Higher oxidation states typically result in a larger energy difference between the d orbitals. Consequently, the energy cost of pairing electrons is relatively high, promoting the high spin configuration.
3. Coordination Number: The coordination number of the complex, which represents the number of ligands directly bonded to the central metal ion, also influences the high spin configuration. Higher coordination numbers provide more available d orbitals for electron occupation. With more orbitals to fill, the likelihood of having unpaired electrons increases, favoring the high spin configuration.
It’s important to note that these factors are interrelated and collectively determine the spin state of a coordination complex. A strong ligand field combined with a high oxidation state and a high coordination number would strongly favor the high spin configuration. Conversely, a weak ligand field, low oxidation state, and low coordination number would tend to promote the low spin configuration with fewer unpaired electrons.
Ligand field strength
Ligand field strength refers to the ability of a ligand to influence the energy levels of the d orbitals of a central metal ion in a coordination complex. It determines the magnitude of the energy difference between the d orbitals, known as the crystal field splitting, which affects the electronic configuration and spin state of the complex.
The ligand field strength depends on several factors, including:
1. Nature of the Ligand: Different ligands have varying abilities to interact with the central metal ion. Ligands can be classified as strong-field ligands or weak-field ligands. Strong-field ligands, such as cyanide (CN-) and carbon monoxide (CO), have a higher ability to interact with the metal ion and cause a larger crystal field splitting. Weak-field ligands, such as water (H2O) and ammonia (NH3), have a lower ability to interact, resulting in a smaller crystal field splitting.
2. Ligand Charge and Size: The charge and size of the ligand also influence the ligand field strength. Ligands with higher charges or smaller sizes have a greater ability to interact with the metal ion and generate a larger crystal field splitting. For example, negatively charged ligands, like chloride (Cl-) or oxalate (C2O4^2-), exert a stronger ligand field than neutrally charged ligands, such as water or pyridine.
3. Ligand Geometry and Symmetry: The geometry and symmetry of the ligand can affect the ligand field strength. Ligands with a more symmetric arrangement around the metal ion generally result in a weaker ligand field. For instance, tetrahedral complexes often have weaker ligand fields compared to octahedral complexes due to the lower degree of asymmetry in their ligand arrangements.
The ligand field strength has significant consequences on the electronic configuration and spin state of the coordination complex. Strong-field ligands cause a larger energy difference between the d orbitals, making it energetically favorable for electrons to pair up, leading to a low spin configuration with fewer unpaired electrons. Weak-field ligands result in a smaller energy difference, promoting the high spin configuration with more unpaired electrons.
Understanding ligand field strength is crucial for predicting and explaining the electronic and magnetic properties of coordination complexes, as well as their reactivity and spectroscopic behavior. It also plays a vital role in the design and development of new coordination compounds for specific applications.
The metal ion oxidation state
The metal ion oxidation state refers to the formal charge of the metal ion in a coordination complex, indicating the number of electrons gained or lost by the metal during the complex formation. It plays a significant role in determining the electronic configuration and spin state of the complex.
The oxidation state of the metal ion influences the energy levels of the d orbitals and their occupancy in the coordination complex. Higher oxidation states typically result in a larger energy difference between the d orbitals, known as crystal field splitting. Consequently, the energy cost of pairing electrons becomes significant, favoring the low spin configuration with fewer unpaired electrons.
When the metal ion has a high oxidation state (e.g., +3, +4, +5), the crystal field splitting is relatively large, and the energy cost of pairing electrons is high. The d orbitals are preferentially filled in a way that maximizes the number of paired electrons, resulting in a low spin configuration.
When the metal ion has a low oxidation state (e.g., +1, +2), the crystal field splitting is smaller, and the energy cost of pairing electrons is relatively low. Consequently, the d orbitals are filled in a way that maximizes the number of unpaired electrons, leading to a high spin configuration.
It’s important to note that the oxidation state of the metal ion is not the sole determining factor of the spin state. Such as ligand field strength and coordination number, also play crucial roles in influencing the spin state of the complex. The interplay between these factors collectively determines whether a complex exhibits a high spin or low spin configuration.
Understanding the metal ion oxidation state is essential for predicting and explaining the electronic structure, magnetic properties, and reactivity of coordination complexes. It also guides the selection of appropriate ligands and conditions for synthesizing specific coordination compounds with desired properties.
What is Low Spin Complexes?
Low spin complexes are coordination complexes in which the electrons occupy the available d orbitals in a way that minimizes the number of unpaired electrons. These complexes are characterized by a lower number of unpaired electrons and a smaller magnetic moment compared to high-spin complexes.
The electrons are paired up in the d orbitals before filling up the higher energy levels. This means that electrons are distributed across the available d orbitals with opposite spins, resulting in a minimum number of unpaired electrons. The pairing of electrons follows Hund’s rule, which states that electrons will occupy separate orbitals with the same spin before pairing occurs.
The spin state of a coordination complex is influenced by several factors, including the strength of the ligand field, the oxidation state of the metal ion, and the coordination number of the complex. Low spin configurations are favored when the ligand field is strong, the metal ion has a lower oxidation state, and/or the coordination number is low.
The presence of fewer unpaired electrons in low-spin complexes has implications for their magnetic properties. Low spin complexes exhibit smaller magnetic moments due to the reduced number of unpaired electrons, which interact less with external magnetic fields compared to high spin complexes.
Examples of low spin complexes include octahedral complexes such as [Co(NH3)6]3+ and tetrahedral complexes such as [FeCl4]2-. These complexes exhibit a low spin configuration due to the presence of a strong ligand field or a lower coordination number.
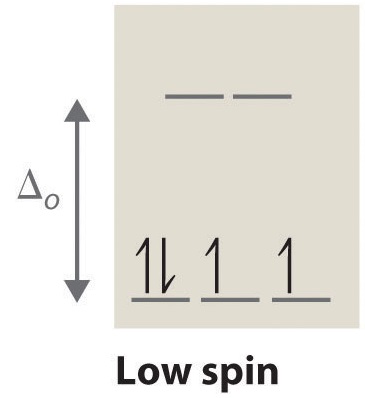
Low spin complexes are coordination complexes with a smaller number of unpaired electrons, resulting in a smaller magnetic moment and distinct properties compared to high spin complexes.
Factors influencing the low spin configuration
Several factors influence the low spin configuration in coordination complexes. These factors determine the extent to which electrons occupy the available d orbitals with opposite spins, resulting in a lower number of unpaired electrons.
The key factors influencing the low spin configuration are:
1. Ligand Field Strength: The ligand field strength plays a crucial role in determining the low spin configuration. Strong ligand fields, such as small and highly charged ligands, cause a larger energy difference between the d orbitals. In such cases, the energy cost of pairing electrons becomes relatively low, favoring the low spin configuration with minimal unpaired electrons. Strong-field ligands, including cyanide (CN-) and carbon monoxide (CO), tend to promote the low spin configuration.
2. Metal Ion Oxidation State: The oxidation state of the central metal ion affects the energy levels of the d orbitals. Lower oxidation states typically result in a smaller energy difference between the d orbitals. Consequently, the energy cost of pairing electrons is relatively low, promoting the low spin configuration. Metal ions with lower oxidation states, such as +1 or +2, are more likely to exhibit a low spin configuration.
3. Coordination Number: The coordination number of the complex also influences the low spin configuration. Lower coordination numbers provide fewer available d orbitals for electron occupation. With fewer orbitals to fill, the likelihood of having unpaired electrons decreases, favoring the low spin configuration.
It’s important to note that these factors are interrelated, and their collective influence determines the spin state of a coordination complex. A strong ligand field combined with a lower oxidation state and a lower coordination number would strongly favor the low spin configuration. Conversely, a weak ligand field, higher oxidation state, and higher coordination number would tend to promote the high spin configuration with more unpaired electrons.
Understanding the factors influencing the low spin configuration is essential for predicting and explaining the electronic structure, magnetic properties, and reactivity of coordination complexes. It also guides the selection of appropriate ligands and conditions for synthesizing specific coordination compounds with desired properties.
Ligand field strength
Ligand field strength refers to the ability of a ligand to influence the energy levels of the d orbitals of a central metal ion in a coordination complex. It determines the magnitude of the energy difference between the d orbitals, known as the crystal field splitting, which affects the electronic configuration and spin state of the complex.
The ligand field strength depends on several factors, including:
1. Nature of the Ligand: Different ligands have varying abilities to interact with the central metal ion. Ligands can be classified as strong-field ligands or weak-field ligands. Strong-field ligands, such as cyanide (CN-) and carbon monoxide (CO), have a higher ability to interact with the metal ion and cause a larger crystal field splitting. Weak-field ligands, such as water (H2O) and ammonia (NH3), have a lower ability to interact, resulting in a smaller crystal field splitting.
2. Ligand Charge and Size: The charge and size of the ligand also influence the ligand field strength. Ligands with higher charges or smaller sizes have a greater ability to interact with the metal ion and generate a larger crystal field splitting. For example, negatively charged ligands, like chloride (Cl-) or oxalate (C2O4^2-), exert a stronger ligand field than neutrally charged ligands, such as water or pyridine.
3. Ligand Geometry and Symmetry: The geometry and symmetry of the ligand can affect the ligand field strength. Ligands with a more symmetric arrangement around the metal ion generally result in a weaker ligand field. For instance, tetrahedral complexes often have weaker ligand fields compared to octahedral complexes due to the lower degree of asymmetry in their ligand arrangements.
The ligand field strength has significant consequences on the electronic configuration and spin state of the coordination complex. Strong-field ligands cause a larger energy difference between the d orbitals, making it energetically favorable for electrons to pair up, leading to a low spin configuration with fewer unpaired electrons. In contrast, weak-field ligands result in a smaller energy difference, promoting the high spin configuration with more unpaired electrons.
Understanding ligand field strength is crucial for predicting and explaining the electronic and magnetic properties of coordination complexes, as well as their reactivity and spectroscopic behavior. It also plays a vital role in the design and development of new coordination compounds for specific applications.
The metal ion oxidation state
The metal ion oxidation state refers to the formal charge of the metal ion in a coordination complex, indicating the number of electrons gained or lost by the metal during the complex formation. It is denoted by a Roman numeral following the metal symbol.
The oxidation state of the metal ion is determined by the number of electrons it has either gained or lost to achieve a stable electronic configuration. It represents the charge that would result if all the ligands in the complex were removed with their electron pairs.
The metal ion oxidation state has significant implications for the electronic structure and reactivity of coordination complexes. It influences the energy levels of the d orbitals and affects the stability, geometry, and magnetic properties of the complex.
Here are a few key points regarding the metal ion oxidation state:
1. Electrons Gained or Lost: The metal ion oxidation state indicates whether the metal ion has gained or lost electrons compared to its neutral state. A positive oxidation state (+n) indicates that the metal ion has lost n electrons, while a negative oxidation state (-n) indicates that it has gained n electrons.
2. Electron Configuration: The metal ion oxidation state determines the electronic configuration of the metal ion within the complex. It influences how the d orbitals of the metal ion are occupied by electrons.
3. Ligand Interactions: The metal ion oxidation state affects the strength of interactions between the metal ion and the ligands. It influences the overall charge distribution in the complex and can impact the ligand’s binding affinity and stability.
4. Redox Chemistry: The metal ion oxidation state plays a crucial role in redox reactions involving coordination complexes. It determines the ability of the metal ion to accept or donate electrons during chemical reactions.
The metal ion oxidation state is often indicated in the name or formula of the coordination complex. It is essential to consider the oxidation state when predicting the properties and behavior of coordination compounds, including their coordination geometry, magnetism, and reactivity.
Comparison Chart
Here’s a comparison chart highlighting the key differences between high-spin and low-spin complexes:
| High Spin Complexes | Low Spin Complexes | |
|---|---|---|
| Electronic Configuration | Maximizes the number of unpaired electrons | Minimizes the number of unpaired electrons |
| Number of Unpaired Electrons | A higher number of unpaired electrons | The lower number of unpaired electrons |
| Magnetic Moment | Higher magnetic moment | Smaller magnetic moment |
| Magnetic Susceptibility | Positive (Paramagnetic) | Negative (Diamagnetic) |
| Ligand Field Strength | Weak ligand field | Strong ligand field |
| Crystal Field Splitting | The relatively small energy difference between d orbitals | The larger energy difference between d orbitals |
| Ligand Type | Weak-field ligands | Strong-field ligands |
| Metal Ion Oxidation State | Higher oxidation state | Lower oxidation state |
| Coordination Number | Higher coordination number | Lower coordination number |
| UV-Vis Spectra | Broad and weak d-d transitions | Sharp and strong d-d transitions |
| EPR Spectra | Larger g-values and broader line widths | Smaller g-values and narrower line widths |
| Temperature Dependence | Weak temperature dependence | Strong temperature dependence |
| Magnetic Susceptibility | Positive | Negative |
| Examples | [Fe(H2O)6]2+ | [Fe(CN)6]3- |
Experimental Techniques and Observations
Experimental techniques and observations are essential for studying and characterizing high-spin and low-spin complexes. Several methods can provide valuable information about the electronic structure, magnetic properties, and spectroscopic behavior of these complexes.
Here are some commonly used experimental techniques and the observations they provide:
1. Magnetic Susceptibility: Magnetic susceptibility measurements can determine whether a complex is paramagnetic (high spin) or diamagnetic (low spin). Paramagnetic complexes exhibit a positive magnetic susceptibility, indicating the presence of unpaired electrons and a high spin configuration. Diamagnetic complexes have a negative magnetic susceptibility, suggesting the absence or minimal presence of unpaired electrons and a low spin configuration.
2. UV-Vis Spectroscopy: Ultraviolet-visible spectroscopy provides information about the electronic transitions in coordination complexes. High-spin and low-spin complexes often exhibit distinct absorption spectra due to the different energies of their d-d transitions. High spin complexes typically show broad and weak d-d transitions, while low spin complexes exhibit sharper and stronger d-d transitions.
3. EPR Spectroscopy: Electron Paramagnetic Resonance (EPR) spectroscopy is a powerful technique for studying paramagnetic complexes. It can determine the number of unpaired electrons and provide information about their environment and magnetic properties. High spin complexes typically show EPR signals with larger g-values and broader line widths, reflecting the presence of multiple unpaired electrons. Low spin complexes exhibit EPR signals with smaller g-values and narrower line widths, indicating fewer unpaired electrons.
4. X-ray Crystallography: X-ray crystallography allows for the determination of the three-dimensional structure of coordination complexes. It provides detailed information about the coordination geometry, bond lengths, and angles within the complex. The structural data obtained from X-ray crystallography can help confirm the electronic configuration and spin state inferred from other experimental techniques.
5. Variable-Temperature Magnetic Measurements: Variable-temperature magnetic measurements can provide insights into the temperature dependence of the magnetic properties of coordination complexes. This technique can help determine the nature of the magnetic interactions and confirm the spin state. High spin complexes generally show weak temperature dependence, while low spin complexes exhibit stronger temperature dependence due to the presence of spin-coupling interactions.
6. Mössbauer Spectroscopy: Mössbauer spectroscopy is a technique used to study the oxidation state and coordination environment of metal ions in complexes. It provides information about the electronic and magnetic properties of the metal center. Mössbauer spectroscopy can reveal subtle changes in the spin state and coordination environment of the metal ion.
These experimental techniques, along with others like NMR spectroscopy and electrochemistry, provide valuable data to characterize and understand the differences between high-spin and low-spin complexes. They allow for the direct observation of electronic states, magnetic properties, and structural features, contributing to a comprehensive understanding of these systems.
Conclusion
The difference between high-spin and low-spin complexes lies in their electron configurations and magnetic properties. High spin complexes maximize the number of unpaired electrons, exhibiting paramagnetic behavior, while low spin complexes minimize the number of unpaired electrons, showing diamagnetic or weak paramagnetic behavior.
Understanding these differences is crucial for unraveling the unique characteristics and applications of transition metal complexes. Further research and exploration in this field will undoubtedly uncover new insights and pave the way for exciting advancements in materials science, catalysis, and other disciplines.
