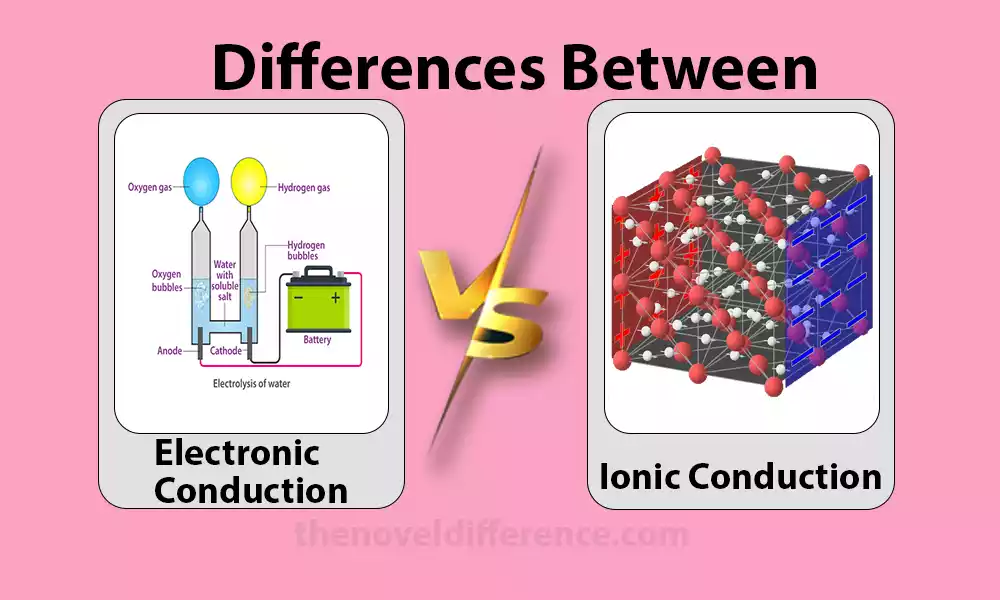
Electronic and ionic conduction are two ways electricity travels through materials. In electronic conduction, electrons, the negatively charged particles within atoms, carry the electrical charge. This type of conduction occurs in conductive materials like metals, where electrons move freely, creating an electric current.
On the other hand, ionic conduction involves the movement of charged ions, atoms with a positive or negative charge, to transmit electricity. Ionic conduction commonly occurs in electrolytes, like solutions or molten salts, where ions move and carry the charge. It’s prevalent in batteries, where ions flow between electrodes, enabling the transfer of electrical energy.
While electronic conduction is prevalent in metals and conductors, ionic conduction is typical in electrolytes and is vital for certain technologies like batteries, fuel cells, and some types of sensors, each playing a unique role in electrical transmission.
Importance of Electronic and Ionic Conduction
Understanding the difference between electronic and ionic conduction is crucial for several reasons:
1. Material Selection: Knowledge of whether a material exhibits electronic or ionic conduction is essential in choosing the appropriate material for specific applications. For instance, electronic conductors like metals are preferred for electrical wiring, whereas ionic conductors (electrolytes) are used in batteries and fuel cells.
2. Device Design and Performance: Electronic and ionic conduction plays a vital role in the design and performance of various electronic devices. Understanding the mechanisms and properties of conduction helps engineers optimize device efficiency and functionality.
3. Energy Storage and Conversion: Ionic conduction is fundamental to energy storage and conversion technologies such as batteries, supercapacitors, and fuel cells. By comprehending the characteristics of ionic conduction, researchers can develop advanced materials and designs to enhance energy storage and conversion efficiency.
4. Electrochemistry: Ionic conduction is critical in electrochemical processes, including corrosion, electroplating, and electrolysis. Understanding the difference between electronic and ionic conduction enables better control and manipulation of these processes.
5. Semiconductor Devices: Electronic conduction is the basis of semiconductor technology. Differentiating Between Electronic and Ionic Conduction Helps in Understanding the Behavior of Semiconductors, Which is Essential for the Design and Operation of Electronic Devices Such as Transistors and Integrated Circuits.
6. Fundamental Scientific Understanding: Studying electronic and ionic conduction provides insights into the behavior of matter at the atomic and molecular levels. It contributes to our understanding of fundamental physical and chemical phenomena and enables advancements in materials science and solid-state physics.
Comprehending the difference between electronic and ionic conduction empowers scientists, engineers, and researchers to make informed decisions, design better devices, and contribute to the development of innovative technologies across various fields.
What is Electronic Conduction?
Electronic conduction refers to the movement of electrons through a material, allowing the flow of electric current. In materials that exhibit electronic conduction, such as metals, the outermost electrons of atoms are loosely bound and can move freely. When a Voltage is Applied Across the Material, the Electrons Gain Energy and Move in Response to the Electric Field, Creating a Flow of charge.
The Mechanism of Electronic Conduction Relies on the Presence of a Large Number of Mobile Electrons That Can Carry Electrical Charge From One Point to Another. These Mobile Electrons are Often Referred to as “Free Electrons” or “Conduction electrons.” Unlike in insulators, Where Electrons Are Tightly Bound to Their Atoms and Cannot Move Easily, the Availability of Free Electrons in Conductive Materials Allows for Efficient Electron Movement and Electrical conductivity.
The Conductivity of a Material for Electronic Conduction Depends on Factors Such as the Number of Free Electrons, Their Mobility, and the Presence of Impurities or Defects in the Material. Metals, Such as Copper and Aluminum, Are Excellent Electronic Conductors Due to Their High Density of Free Electrons. Electronic conduction is fundamental in various applications, including electrical wiring, electronic devices, and circuitry.
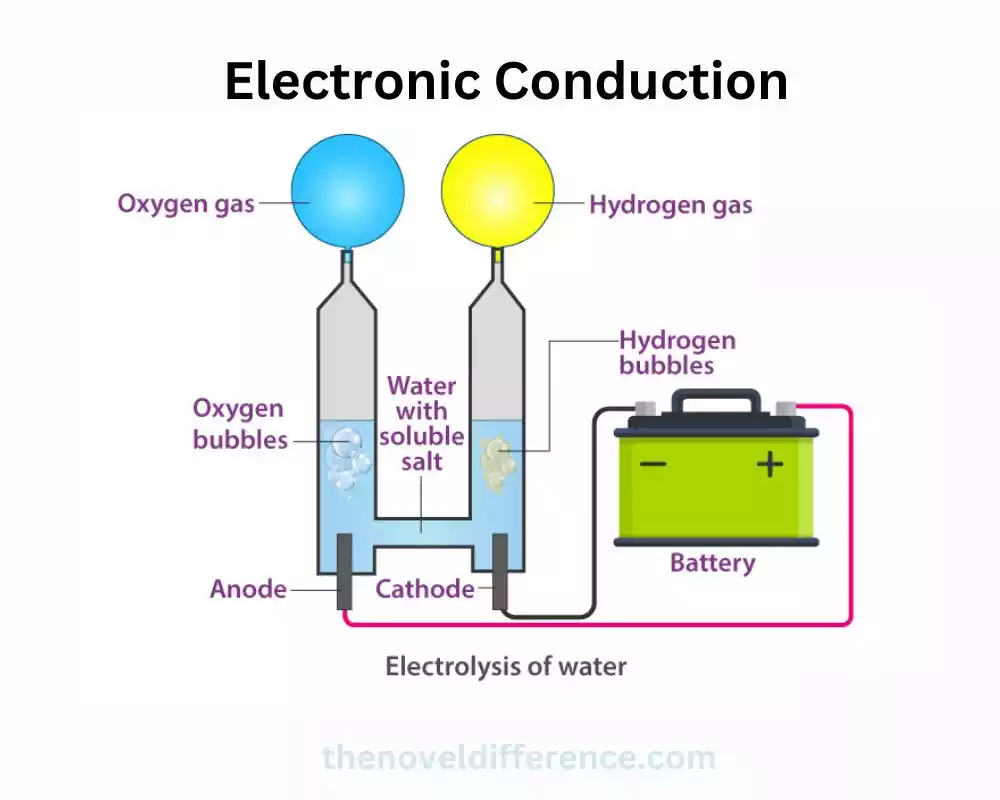
Conductors and insulators
Conductors and insulators are two categories of materials that exhibit different levels of electrical conductivity:
1. Conductors: Conductors are materials that have high electrical conductivity, meaning they allow electric charges to flow easily through them. In conductors, the outermost electrons of atoms are loosely bound, enabling them to move freely within the material. This movement of electrons facilitates the transfer of electric current. Metals, Such as Copper, Silver, and Gold, Are Excellent Conductors of Electricity Due to Their High Concentration of Free Electrons. Other materials, like graphite and some solutions (e.g., saline water), also exhibit good conductivity.
2. Insulators: Insulators, also known as non-conductors, are materials that have very low electrical conductivity. In insulators, electrons are strongly bound to their respective atoms and are not able to move freely. As a result, electric charges cannot flow easily through these materials. Insulators are commonly used to prevent the leakage of electricity and to provide electrical insulation. Examples of insulators include Rubber, Glass, Plastic, Ceramics, and Dry wood.
The Classification of Materials as Conductors or insulators is Based on Their inherent Electronic Structure and the Ability of Their Electrons to Move in Response to an Electric Field. It’s important to Note That the Distinction Between Conductors and insulators is Not Always Black and White, as Some Materials May Exhibit Intermediate Conductivity Levels and Are Referred to as Semiconductors. Semiconductors, like silicon and germanium, have an electrical conductivity that can be controlled and modified by doping or applying external factors, making them key components in electronic devices like transistors.
Role of electrons in electronic conduction
Electrons play a fundamental role as the charge carriers responsible for the flow of electric current through a material.
Here are the key roles of electrons in electronic conduction:
1. Charge Carriers: Electrons are negatively charged particles orbiting the atomic nucleus. These free electrons are the primary charge carriers. When a Voltage is Applied Across a Conductive Material, the Electric Field Exerts a Force on the Free Electrons, Causing Them to Move in Response to the Field.
2. Electron Mobility: The mobility of electrons refers to their ability to move through a material in response to an electric field. The conductivity of a material for electronic conduction is influenced by the mobility of its free electrons. Materials with high electron mobility, such as metals, exhibit high electrical conductivity.
3. Energy Transfer: As electrons move through a conductor, they transfer energy in the form of electrical current. This energy transfer allows for the transmission of power and electrical signals in various electronic devices and systems.
4. Role in Electric Circuits: Electrons enable the flow of electric current within electric circuits. When a Voltage is Applied Across a Circuit, Electrons Move From the Negative Terminal of the Power Source (Cathode) Towards the Positive Terminal (Anode), Creating an Electric Current. The movement of electrons allows for the transfer of electrical energy to power devices or perform work.
5. Role in Semiconductor Devices: In semiconductor devices like transistors and diodes, the control and manipulation of electron flow are essential. The behavior of electrons in semiconductors, including their movement and interactions, determines the functionality and characteristics of these devices.
Understanding the behavior and properties of electrons in electronic conduction is crucial for designing and optimizing electronic devices, circuits, and systems. It forms the basis of electronics and enables the transmission and control of electrical energy in various technological applications.
What is Ionic Conduction?
Ionic conduction refers to the movement of ions through a material, allowing the flow of electric current. Unlike electronic conduction, which involves the movement of electrons, ionic conduction relies on the migration of positively or negatively charged ions.
In materials that exhibit ionic conduction, such as electrolytes, ions are mobile and able to move through the material when a voltage is applied. Ionic conduction typically occurs in materials that are composed of ions, such as dissolved salts, molten salts, and certain types of ceramics. These materials are often referred to as ionic conductors or electrolytes.
When a Potential Difference is Applied Across the Material, the Positive Ions (Cations) Migrate Toward the Negatively Charged Electrode (Cathode), While the Negative Ions (Anions) Move Toward the Positively Charged Electrode (Anode). This movement of ions creates an electric current.
The conductivity of an ionic conductor depends on factors such as the concentration of mobile ions, their mobility, temperature, and the presence of defects or impurities in the material. Ionic conductors are widely used in various applications, including batteries, fuel cells, sensors, and electrolytic processes.
It is worth noting that while electronic conduction occurs in a wide range of materials, ionic conduction is more limited and typically associated with materials specifically designed or engineered to have mobile ions and enable ion transport.
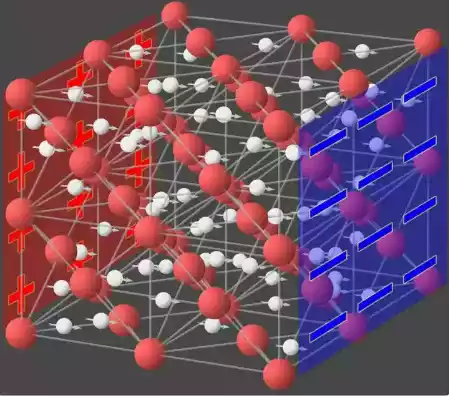
Role of ions in ionic conduction
Ions play a crucial role in ionic conduction. They are responsible for carrying electric charge and facilitating the flow of electric current in materials that exhibit ionic conduction.
Here are the key roles of ions in this process:
1. Charge Carriers: Ions act as charge carriers in ionic conductors. Positive Ions (Cations) and Negative Ions (Anions) Are Free to Move Within the Material. When an Electric Field Is Applied, the Cations Move Toward the Negatively Charged Electrode (Cathode), While the ions move Toward the Positively Charged Electrode (Anode). This movement of ions creates the flow of electric current.
2. Migration: Ions in an ionic conductor migrate from one location to another in response to the applied electric field. The migration of ions is driven by the attractive and repulsive forces between the charged ions and the electrodes. This movement of ions allows for the transfer of charge and the propagation of electric current through the material.
3. Electrolysis: Ions are essential for chemical reactions to occur. For example, in electroplating, positive metal ions from a solution migrate toward the negatively charged object to be plated, where they are reduced to form a metal coating. Similarly, in electrolysis, ions are involved in reactions at the electrodes, allowing for the decomposition of compounds or the deposition of desired materials.
4. Ion Mobility: The mobility of ions, or their ability to move within the material, is a crucial factor in determining the conductivity of an ionic conductor. Factors such as ion size, charge, and the nature of the material influence ion mobility. Higher ion mobility leads to greater ionic conductivity.
By their charges and mobility, ions enable the transport of electric charge and the conduction of electricity in materials that exhibit ionic conduction. This phenomenon is fundamental to the functioning of various devices and systems, including batteries, fuel cells, electrochemical sensors, and electrolytic processes.
Factors affecting ionic conductivity
Several factors can affect the conductivity of ions in materials that exhibit ionic conduction. These factors influence the mobility and behavior of ions, thus impacting the overall ionic conductivity of the material.
Here are the key factors affecting ionic conductivity:
1. Ion Concentration: The concentration of mobile ions in the material has a significant impact on its ionic conductivity. Higher ion concentration generally leads to higher conductivity. Increasing the concentration of ions increases the likelihood of ion-ion collisions and facilitates faster ion transport.
2. Ion Mobility: The mobility of ions, or their ability to move within the material, is a critical factor in determining ionic conductivity. Factors That Affect Ion Mobility Include the Size and Charge of the Ions, the Presence of Defects or impurities in the Material, and the Nature of the Ion-Ion interactions. Smaller ions and ions with higher charges generally exhibit higher mobility.
3. Temperature: Temperature has a significant influence on ionic conductivity. In general, as temperature increases, so does the ionic conductivity. This is because higher temperatures provide more thermal energy, allowing ions to overcome energy barriers and move more easily through the material.
4. Type of Ions: Different ions may have varying degrees of mobility and interaction with the surrounding material. The specific properties of the ions, such as their size, charge, and hydration shell, can affect their ability to move and contribute to ionic conductivity.
5. Presence of Impurities and Defects: Impurities and defects in the material’s crystal structure can impact ionic conductivity. These defects can create additional sites for ion migration or introduce barriers to ion movement, affecting the overall conductivity of the material.
6. Material Structure and Composition: The structure and composition of the material play a crucial role in determining its ionic conductivity. Factors such as crystal structure, grain boundaries, and the presence of crystalline defects can influence ion mobility and affect the overall conductivity.
Understanding These Factors and Their Influence on Ionic Conductivity is important for Designing and Optimizing Materials for Applications Such as Batteries, Fuel Cells, Electrolytes, and Other Ionic Devices. It allows researchers to tailor material properties and conditions to achieve desired levels of ionic conductivity for specific applications.
Differences between Electronic and Ionic Conduction
Electronic and ionic conduction are two distinct mechanisms for the flow of electric current in materials.
Here are the key differences between electronic and ionic conduction:
1. Nature of Charge Carriers:
• Electronic Conduction: The charge carriers in electronic conduction are electrons, which are negatively charged particles orbiting the atomic nucleus.
• Ionic Conduction: The charge carriers in ionic conduction are ions, which are atoms or molecules that have gained or lost electrons, resulting in a positive or negative charge.
2. Mechanisms of Conduction:
• Electronic Conduction: Electric current flows through the movement of electrons in a material. The loosely bound outermost electrons in conductive materials can move freely in response to an electric field.
• Ionic Conduction: Ionic conduction involves the movement of ions through a material. When an Electric Field is Applied, Positive Ions (Cations) Move Toward the Negative Electrode, and Negative Ions (Anions) Move Toward the Positive Electrode.
3. Types of Materials Involved:
• Electronic Conduction: Electronic conduction occurs in materials with a high concentration of free electrons, such as metals and certain semiconductors.
• Ionic Conduction: Ionic conduction occurs in materials that contain mobile ions, such as electrolytes, dissolved salts, and certain ceramics.
4. Speed of Conduction:
• Electronic Conduction: Electronic conduction generally occurs at higher speeds because electrons are relatively lightweight and can move rapidly through materials.
• Ionic Conduction: Ionic conduction typically occurs at slower speeds compared to electronic conduction. The larger size and greater mass of ions result in slower movement through the material.
5. Factors Affecting Conductivity:
• Electronic Conduction: The conductivity of electronic conductors depends on factors such as temperature, impurities, and the density of free electrons in the material.
• Ionic Conduction: Ionic conductivity is influenced by factors such as the concentration of mobile ions, their mobility, temperature, and the presence of defects or impurities in the material.
6. Applications and Significance:
• Electronic Conduction: Electronic conduction is essential in various fields, including electronics, electrical wiring, integrated circuits, and semiconductor devices.
• Ionic Conduction: Ionic conduction plays a crucial role in applications such as batteries, fuel cells, electrolysis, electrochemical sensors, and certain types of chemical reactions.
Understanding the differences between electronic and ionic conduction is essential for selecting suitable materials, designing devices, and optimizing performance in various technological and scientific applications.
Similarities between Electronic and Ionic Conduction
While electronic and ionic conduction has distinct mechanisms and charge carriers.
There are some similarities between Electronic and Ionic Conduction:
1. Movement of Charged Particles: Both electronic and ionic conduction involve the movement of charged particles through a material. Electrons Carry the Electric Charge, While in Ionic Conduction, Ions Carry the Charge.
2. Transfer of Electric Current: Both electronic and ionic conduction facilitate the flow of electric current. In both cases, the movement of charged particles allows for the transfer of electric charge from one point to another.
3. Importance in Various Fields: Both electronic and ionic conduction play vital roles in different scientific and technological fields. They are essential in areas such as electronics, energy storage and conversion, electrochemistry, and semiconductor devices.
4. Influence of Factors on Conductivity: The conductivity of both electronic and ionic conductors can be affected by various factors. Factors Like Temperature, Impurities, and Defects can impact the Conductivity of Electronic Conductors, While Factors Such as Ion Concentration, Mobility, and Temperature Can influence the Conductivity of Ionic Conductors.
5. Electrical Applications: Both electronic and ionic conduction find applications in electrical devices and systems. Electronic conduction is integral to the functioning of electronic circuits, wiring, and electrical components. Ionic conduction is crucial in batteries, fuel cells, electrolytic processes, and electrochemical sensors.
While there are differences in the mechanisms and charge carriers involved, recognizing the similarities between electronic and ionic conduction can contribute to a broader understanding of conduction phenomena and their applications.
The final word
Understanding the differences between electronic and ionic conduction is vital for comprehending their distinct roles in different contexts. While electronic conduction relies on the movement of electrons in conductive materials, ionic conduction involves the migration of ions in electrolytes.
These contrasting mechanisms, properties, and applications contribute to the diverse range of functions each mode of conduction fulfills. Whether it’s in the realm of electronics or electrolytes, the dissimilarities between electronic and ionic conduction play a significant role in shaping technological advancements and scientific understanding.