Overview of Glyceraldehyde and Glycerate
Glyceraldehyde – Overview:
Glyceraldehyde is a member of the aldehyde family. It is a monosaccharide that has three carbons. Glyceraldehyde is an important component of many metabolic pathways. This is an essential component of glycolysis which breaks down glucose to produce energy. The two main forms of glyceraldehyde are D and L.
Glycerate – Overview:
Glycerate is also called glyceric acid. It is a three-carbon organic compound that comes from glycerol. It is classified as sugar acid, and it is involved in carbon fixation during photosynthesis. Glycerate is a key component of the Calvin cycle.
This is a series of reactions in which carbon dioxide in plants becomes organic compounds. Glycerate is also available in two different forms, D-glycerate and L-glycerate.
Although both glyceraldehyde (glycerate) and glycerate play important roles in biological systems, they are different in terms of their structure, metabolism, and physiological functions. Understanding the differences between these molecules is essential for understanding their diverse roles in biochemical processes.
Understanding the differences between the two
There are many reasons to know the difference between Glyceraldehyde and Glycerate:
- Metabolic pathways: Both glyceraldehyde and glycerate are involved in different metabolic pathways. Glyceraldehyde plays a crucial role in glycolysis, which is essential for the breakdown of glucose. Glycerate is linked to the Calvin Cycle during photosynthesis, where it helps fix carbon. Understanding these distinct pathways will help us to better understand how organisms use carbohydrates and produce energy.
- Functional Meaning: Glyceraldehyde and glycerate each serve specific physiological roles. Glyceraldehyde is essential in energy metabolism as well as cell processes like carbohydrate and lipid metabolism; it plays a key role in synthesizing ATP (the currency for cells). Glycerate helps plants fix carbon into organic compounds necessary for plant growth. By understanding their individual functions in biological systems, one will gain more insight into their roles within biological environments.
- Biochemical Reactions: Glyceraldehyde and glycerate both play an essential role in biochemical reactions. Glyceraldehyde is especially implicated in glycolytic processes that result in the creation of pyruvate. This leads to energy production. Glycerate is involved in the Calvin cycle enzymatic reaction, which leads to the synthesis of glucose and other organic molecules. Understanding the reactions and enzymes that are associated with glyceraldehyde, and glycerate, can provide insight into the biochemical processes taking place in cells.
- Applications and Industries: Understanding the difference between Glyceraldehyde and Glycerate is useful for many industries. Glyceraldehyde can be used as a biochemical reagent and a precursor in the biotechnological, pharmaceutical, and biochemical industries. Its role in carbon fixation and plant metabolism makes it a useful ingredient in agriculture and biofuels. Understanding their differences will help to optimize the application of these products and create innovative solutions.
Understanding the differences between Glyceraldehyde (Glycerate) and Glyceraldehyde is important for understanding their metabolic pathways, functions, biochemical reactions and applications in different industries. It helps to understand their roles in photosynthesis and energy metabolism.
Definition and Structure
Structure and Definition of Glyceraldehyde:
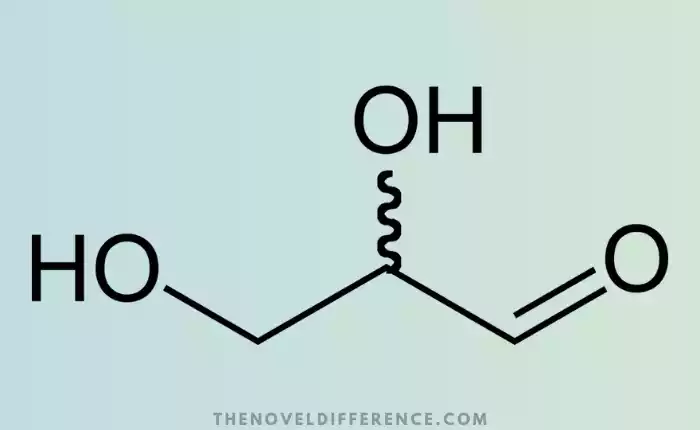
Glyceraldehyde, also known as monosaccharide or simple organic compound C3H6O3, is a simple and straightforward organic compound. It belongs to the aldehyde class, which is characterized by a carbonyl (-CHO), at the end of carbon chains.
It is a straight-chain sugar that has a structure of three carbons. It is available in two stereoisomeric versions: D-glyceraldehyde, also known as (+)-glyceraldehyde, and L-glyceraldehyde, also known as (-)-glyceraldehyde. The arrangement of hydroxyl groups in these isomers varies. D-glyceraldehyde, the natural form, is found more frequently in biological systems.
Glyceraldehyde is composed of three hydroxyl groups, an aldehyde (-CHO), and a central carbon (C2). The carbon atoms that are adjacent to the central carbon atom are designated as C1 and C3. Multiple hydroxyl groups make glyceraldehyde a polar molecule that can form hydrogen bonds.
Structure and Definition of Glycerate:
Glycerate is also called glyceric acid. It has a chemical formula of C3H6O4. The oxidation of one of the hydroxyl groups in glycerol (trihydroxy alcohol) produces glycerate.
The two forms of glycerate are D-glycerate (naturally occurring) and L-glycerate. They differ by the way the hydroxyl group is arranged around the asymmetrical carbon atom. D-glycerate, which is similar to glyceraldehyde in its natural form, is also the most common.
Glycerate is a type of glyceride compound composed of three hydroxyl (-OH) groups, one carboxyl (-COOH) group, and a hydrogen atom; adjacent carbon atoms may also be designated C1 or C3, creating a polar compound.
Glyceraldehyde, on the other hand, is a sugar of three carbons with an aldehyde, while glycerate, which is derived from glycerol, is a sugar acid of three carbons.
Both molecules are stereoisomeric and have a different arrangement of hydroxyl groups around the central carbon. Understanding their definitions, structures, and functions is essential to explore their properties and biochemical roles.
Biosynthesis and Metabolism
Glyceraldehyde Biosynthesis and Metabolism:
- Role In Glycolysis: Glyceraldehyde Is A Key Intermediate In The Glycolysis Pathway. Glyceraldehyde plays a major role in the glycolysis process, which is metabolically the process of breaking down glucose into energy. In the initial steps of glycolysis, glucose is converted to glyceraldehyde-3-phosphate through a series of enzymatic reactions. Glyceraldehyde-3-phosphate then undergoes further reactions to produce pyruvate, generating ATP and NADH in the process. Pyruvate is further metabolized by various pathways including aerobic respiration and fermentation to produce more energy.
- Pathways of Biosynthesis: Multiple pathways can be used to synthesize glyceraldehyde in the body. One of the main pathways involves the breakdown of glucose-6-phosphate. This is an intermediate in the glucose metabolism. Glucose-6-phosphate is converted to fructose-6-phosphate, which is then converted to glyceraldehyde-3-phosphate through a series of enzymatic reactions.
- Metabolic Fate & Functions: Glyceraldehyde plays a vital role in the metabolism of cells. It is involved with glycolysis which produces ATP, the primary currency for cells. It is used to prepare other molecules, such as glycerol that is necessary for the production of lipids and triglycerides.
Glycerate Biosynthesis and Metabolism:
- Role of Glycerate in the Calvin Cycle: The Calvin Cycle is a metabolic pathway that occurs in chloroplasts in plants and algae. Carbon fixation is achieved by the Calvin cycle, which converts atmospheric carbon dioxide into organic compounds such as glucose. Glycerate is produced during the reduction phase of the Calvin cycle when carbon dioxide is converted into glyceraldehyde-3-phosphate.
- Biosynthesis: Glycerate can be produced via various metabolic pathways. One such pathway is called Calvin Cycle where 3-phosphoglycerate produced during carbon fixing can be reduced into glycerate using an enzyme known as Glyceraldehyde-3-Phosphate dehydrogenase.
- Metabolic Fate & Functions: The Calvin cycle uses glycerate to create organic compounds such as glucose and other carbohydrate. These compounds are used as building blocks and energy sources for the growth and development of plants. Glycerate is also capable of being converted into 3-phosphoglycerate in order to continue carbon fixation. Glycerate is also involved in metabolic pathways that lead to other molecules such as amino acids.
Glyceraldehyde is essential for glycolysis and energy metabolism. Meanwhile, glycerate plays an important role in the Calvin cycle during photosynthesis. Understanding their biosynthesis pathways, as well as their metabolic fate, is essential for understanding the overall metabolism and roles of carbon dioxide and glucose.
Glyceraldehyde & Glycerate comparison chart
Here’s a comparison chart between Glyceraldehyde and Glycerate:
| Aspect | Glyceraldehyde | Glycerate |
|---|---|---|
| Chemical Formula | C3H6O3 | C3H6O4 |
| Structure | Simplest triose sugar | Hydroxy acid derived from glycerol |
| Carbon Atoms | 3 carbon atoms | 3 carbon atoms |
| Functional Groups | Aldehyde group (CHO) | Carboxyl group (COOH) |
| Role in Metabolism | Intermediate in glycolysis | Part of the glycolytic pathway |
| Importance | Essential in carbohydrate metabolism | Intermediate in several metabolic pathways |
| Biochemical Relevance | Key in energy production | Important in gluconeogenesis and glycolysis |
| Oxidation State | Aldehyde state | Carboxylic acid state |
| Example Reaction | Involved in the reduction of NAD+ to NADH in glycolysis | Converted to phosphoglycerate in glycolysis |
Keep in mind that this comparison highlights some of the key differences and roles of glyceraldehyde and glycerate, but the specific context and pathways can lead to variations in their functions and importance.
What are the Similarities between Glyceraldehyde and Glycerate?
While glyceraldehyde & glycerate are distinct, they share many similarities:
- Three-Carbon Structure: Glyceraldehyde, as well as glycerate, are both three-carbon compounds. These compounds are made from glycerol which is a three-carbon compound.
- Hydroxyl groups: Both molecules contain hydroxyl group (-OH). Glyceraldehyde contains three hydroxyls, one for each carbon atom. Glycerate also has three hydroxyls, plus a carboxyl (-COOH) group.
- Stereoisomeric Forms: Glyceraldehyde as well as glycerate are available in two stereoisomeric forms. D- and L’isomers are available, with a difference in the arrangement of the hydroxyl groups surrounding the chiral carbon.
- Importance of Metabolic Pathways: Both compounds are important in metabolic pathways. Glyceraldehyde, a key intermediary in the glycolysis path, contributes to energy production. Glycerate plays an important role in the Calvin Cycle during photosynthesis. It contributes to carbon fixation as well as the synthesis of organic compounds.
- Molecules that can be used as Precursors: Glyceraldehyde and glycerate are both precursors to other molecules. Glyceraldehyde is used to synthesize molecules like glycerol which is essential for lipid metabolism. The Calvin cycle can produce glucose and other carbohydrate molecules from glycerate.
Although there are differences between glyceraldehyde (glycerate) and glyceraldehyde, their similarities highlight their importance as components in metabolic processes and biochemical pathways.
The conclusion of the article
Glyceraldehyde is a monosaccharide involved in glycolysis and energy metabolism. Glyceraldehyde, a monosaccharide, is involved in glycolysis and energy metabolism. Glycerate is an acid sugar that participates in the Calvin Cycle and carbon fixation in photosynthesis.
Glyceraldehyde, and glycerate, share many similarities despite their differences. Both have three carbons atoms and hydroxyl groups. They also exist in D-and L-isomeric forms. Both compounds are also important in metabolic pathways. They serve as precursors to other molecules and they contribute to the production of energy and organic compounds.
Understanding the differences and similarities of glyceraldehyde and glycerate will help you to understand their roles in metabolism, energy generation, and carbon fixing. This knowledge provides insight into the various functions of these compounds and helps unravel the complex biochemical processes that occur in cells.
