When it comes to understanding the behavior of particles and their intelligence, the concept of dipoles plays a imperative part. We will delve into the intriguing world of dipoles, specifically focusing on the difference between induced dipoles and permanent dipoles. Whether you are an understudy examining chemistry or an inquisitive person looking to grow your information, this comprehensive direct will give you essential experiences in this intriguing subject.
Definition of Induced Dipole and Permanent Dipole
Induced Dipole: An induced dipole refers to a temporary separation of charge within a molecule or atom that occurs in the presence of an external electric field. It arises due to the distortion of electron density around the atom or molecule. When an electric field is connected, the adversely charged electrons inside the iota or particle are pulled in or repulsed, driving to a brief partition of positive and negative charges, in this way making an actuated dipole minute. Once the external electric field is removed, the induced dipole disappears, and the molecule returns to its original state.
Permanent Dipole: A permanent dipole refers to a permanent separation of charge within a molecule or atom due to the uneven distribution of electrons. It arises when there is a significant electronegativity difference between atoms within a molecule, resulting in the unequal sharing of electrons. As a result, one conclusion of the particle gets to be marginally more adversely charged (δ-) whereas the other conclusion gets to be somewhat more emphatically charged (δ+), making a lasting dipole minute. The separation of charges is a characteristic property of the molecule and remains even in the absence of an external electric field.
Importance of understanding the difference between the Induced Dipole and Permanent Dipole
Understanding the difference between induced dipole and permanent dipole is important for several reasons:
1. Molecular Interactions: The presence of induced or permanent dipoles in molecules significantly influences their interactions with other molecules. Induced dipoles, for example, play a crucial role in intermolecular forces such as London dispersion forces, which are temporary attractions between molecules. Lasting dipoles can result in dipole-dipole intuitive, which is more grounded and more diligent. Understanding these intuitive is basic in areas such as chemistry, material science, and materials science.
2. Solvent-Solute Interactions: Understanding the difference between induced and permanent dipoles helps explain solubility and solvation processes. Solvents with permanent dipoles, such as water, can interact favorably with solutes that have permanent dipoles or ionic charges. Actuated dipoles in nonpolar solvents, like hydrocarbons, can encourage the disintegration of nonpolar solutes. This information is imperative in areas like pharmacology, natural science, and chemical building.
3. Material Properties: The presence of induced or permanent dipoles can affect the properties of materials. For example, in polymers, the alignment of permanent dipoles within the polymer chains can influence their electrical conductivity, dielectric properties, and optical behavior. Understanding these impacts is pivotal for the planning and advancement of materials for different applications, counting hardware, optics, and vitality capacity.
4. Molecular Modeling and Simulation: Understanding the difference between induced and permanent dipoles is essential in molecular modeling and simulations. Incorporating accurate representations of dipole moments helps in predicting the behavior of molecules, understanding their structures, and simulating their interactions with other molecules or external fields. This knowledge is valuable in fields like drug discovery, material design, and computational chemistry.
5. Biological Systems: Induced and permanent dipoles play important roles in various biological processes. For instance, the interaction between biomolecules such as enzymes, proteins, and DNA relies on the recognition of induced and permanent dipoles. Understanding these interactions is crucial in fields like biochemistry, pharmacology, and molecular biology.
Understanding the difference between induced dipole and permanent dipole is crucial for comprehending molecular interactions, solvation processes, material properties, molecular modeling, and biological systems. This knowledge has broad applications across several scientific disciplines and is essential for advancements in various fields of research and technology.
What is Induced Dipole?
An induced dipole refers to a temporary separation of charge within a molecule or atom that occurs in the presence of an external electric field. It arises due to the distortion of electron density around the atom or molecule. When an electric field is connected, the adversely charged electrons inside the particle or atom are pulled in or repulsed, driving to a brief partition of positive and negative charges, in this way making an actuated dipole minute.
The arrangement of an actuated dipole may be a result of the interaction between the outside electric field and the electron cloud encompassing the particle or atom. The electric field causes a displacement of the electron cloud, creating an imbalance of charges. This temporary separation of charges induces a dipole moment, with one end of the molecule becoming slightly more positive (δ+) and the other end becoming slightly more negative (δ-).
Induced dipoles are inherently temporary. Once the external electric field is removed, the electron cloud returns to its original configuration and the induced dipole moment disappears. This implies that the size and heading of the initiated dipole minute alter with the nearness or nonappearance of the outside electric field.
Induced dipoles play a significant role in intermolecular forces, particularly London dispersion forces. These forces arise due to temporary fluctuations in electron density and the subsequent induction of dipoles in neighboring molecules. London dispersion forces are the primary intermolecular force in nonpolar molecules, and they contribute to various phenomena, including boiling points, solubilities, and molecular interactions.
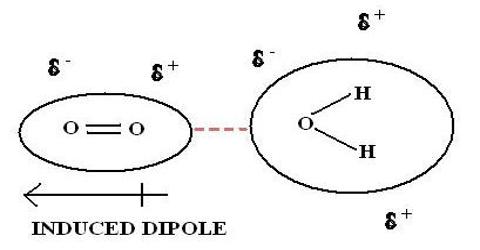
Understanding induced dipoles is important in fields such as chemistry, physics, materials science, and molecular biology, as they have implications for molecular interactions, solvation processes, material properties, and biological systems.
How induced dipoles are formed
Induced dipoles are formed due to the interaction between an external electric field and the electron cloud surrounding an atom or molecule.
The process involves the following steps:
1. Application of an Electric Field: An external electric field is applied to the atom or molecule. The electric field can be created by an electrically charged object or through the application of an electric potential.
2. Interaction with the Electron Cloud: The electric field interacts with the electron cloud surrounding the atom or molecule. The negatively charged electrons experience a force in the presence of the electric field.
3. Electron Displacement: The electric field causes a displacement of the electron cloud. The electrons are either pulled in toward the positive conclusion of the electric field or repulsed from it, depending on the course of the field.
4. Temporary Separation of Charges: The displacement of the electron cloud results in a temporary separation of positive and negative charges within the atom or molecule. One conclusion of the molecule or atom gets to be somewhat more positive (δ+), whereas the other conclusion gets to be somewhat more negative (δ-).
5. Induced Dipole Moment: The separation of charges leads to the formation of an induced dipole moment. The magnitude of the dipole moment depends on the strength of the electric field and the polarizability of the atom or molecule.
It is important to note that induced dipoles are temporary. Once the external electric field is removed, the electron cloud returns to its original configuration and the induced dipole moment disappears. This implies that the size and heading of the initiated dipole minute can alter powerfully in reaction to changes within the outside electric field.
The formation of induced dipoles is a fundamental process underlying various phenomena, such as intermolecular forces, solvation processes, and the behavior of materials in electric fields. It plays a significant role in understanding molecular interactions and properties in numerous scientific disciplines.
Temporary nature of induced dipoles
Induced dipoles are inherently temporary. They emerge due to the nearness of an outside electric field, and their greatness and heading can alter with the nearness or nonattendance of the field.
Here are some key points regarding the temporary nature of induced dipoles:
1. Dependency on External Electric Field: Induced dipoles exist only in the presence of an external electric field. When the field is applied, it causes a displacement of the electron cloud, resulting in the separation of charges and the formation of the induced dipole moment. However, once the external electric field is removed, the electron cloud returns to its original configuration, and the induced dipole moment disappears.
2. Dynamic Response: Induced dipoles exhibit a dynamic response to changes in the external electric field. As the strength or direction of the field varies, the induced dipole moment can adjust accordingly. This means that the magnitude and direction of the induced dipole moment can change in real time, reflecting the changes in the external field.
3. Temporary Charge Separation: The temporary separation of charges within the atom or molecule creates an electric dipole moment. One conclusion of the particle or atom gets to be somewhat more positive (δ+), whereas the other conclusion gets to be somewhat more negative (δ-). This charge separation is not permanent and dissipates when the external electric field is removed.
4. Transient Nature of Intermolecular Interactions: Induced dipoles are responsible for temporary intermolecular interactions, such as London dispersion forces. These interactions arise due to temporary fluctuations in electron density and the subsequent induction of dipoles in neighboring molecules. The dynamic nature of induced dipoles contributes to the transient and temporary nature of intermolecular forces.
5. Reversible Process: The formation and disappearance of induced dipoles are reversible processes. When the external electric field is applied again, the electron cloud responds by creating a new induced dipole moment. This reversibility allows for dynamic and adaptable behavior in molecular systems.
Understanding the temporary nature of induced dipoles is crucial in various fields, such as chemistry, physics, and materials science. It helps explain intermolecular forces, solvation processes, and the behavior of materials in the presence of electric fields.
What is Permanent Dipole?
A permanent dipole refers to a permanent separation of charge within a molecule or atom due to the uneven distribution of electrons. Not at all like initiated dipoles, which are transitory and emerge within the nearness of an outside electric field, permanent dipoles exist indeed within the nonattendance of an outside field.
Here are some key points regarding permanent dipoles:
1. Unequal Sharing of Electrons: Permanent dipoles arise when there is a significant electronegativity difference between atoms within a molecule. Electronegativity is a measure of an atom’s ability to attract electrons toward itself in a chemical bond. When atoms with different electronegativities are bonded, the electrons are not equally shared, resulting in an uneven distribution of electron density.
2. Separation of Charges: Due to the unequal sharing of electrons, one end of the molecule becomes slightly more negatively charged (δ-) while the other end becomes slightly more positively charged (δ+). This charge separation leads to the formation of a permanent dipole moment within the molecule.
3. Origin of Permanent Dipoles: Permanent dipoles can arise from various molecular characteristics, such as polar covalent bonds or molecular asymmetry. The electronegative molecule pulls in electrons more emphatically, coming about in a fractional negative charge on that particle and a fractional positive charge on the other iota. The arrangement of atoms creates an imbalance in the distribution of charges, leading to a permanent dipole moment.
4. Persistence in the Absence of an External Field: Unlike induced dipoles, permanent dipoles persist even when no external electric field is applied. The separation of charges and the resulting dipole moment remain unchanged unless the molecule undergoes a chemical reaction or experiences a change in its electronic structure.
5. Interactions with External Electric Fields: Permanent dipoles can interact with external electric fields. When an electric field is applied, permanent dipoles tend to align themselves with the field. Depending on the introduction of the dipole relative to the field, it can encounter either an appealing or ghastly drive.

Permanent dipoles play a significant role in various phenomena, including dipole-dipole interactions, solvation processes, and the behavior of polar molecules in electric fields. Understanding permanent dipoles is crucial in fields such as chemistry, biology, and materials science, as they influence molecular properties, molecular interactions, and the behavior of substances.
Origin of permanent dipoles in molecules
Permanent dipoles in molecules can arise from different factors, including polar covalent bonds, molecular asymmetry, and resonance effects.
Here are some common origins of permanent dipoles:
1. Polar Covalent Bonds: Permanent dipoles often originate from polar covalent bonds. A polar covalent bond happens when there’s a noteworthy contrast in electronegativity between two reinforced iotas. Electronegativity is the degree of an atom’s capacity to draw in electrons in a chemical bond. The iota with higher electronegativity pulls the shared electrons closer to itself, making a fractional negative charge (δ-) on that molecule and a fractional positive charge (δ+) on the other iota. This charge separation within the bond leads to the formation of a permanent dipole.
2. Molecular Asymmetry: Molecules with an asymmetric distribution of atoms can have a permanent dipole moment. This occurs when the arrangement of atoms within the molecule creates an imbalance in the distribution of charges. For example, a molecule with a bent or V-shaped geometry, such as water (H2O), has a permanent dipole due to the unequal distribution of electron density around the central atom (oxygen). The oxygen atom attracts the shared electrons more strongly than the hydrogen atoms, resulting in a permanent dipole moment with the oxygen end being slightly negative and the hydrogen end being slightly positive.
3. Resonance Effects: Molecules with resonance structures can exhibit a permanent dipole moment. Resonance occurs when a molecule can be represented by multiple Lewis structures with different electron distributions but the same overall connectivity. The resonance structures contribute to the delocalization of electrons, resulting in a partial positive and partial negative charge distribution within the molecule. This charge separation leads to the presence of a permanent dipole moment.
4. Functional Groups: Certain functional groups in organic compounds can contribute to permanent dipoles. For example, the carbonyl group (C=O) found in aldehydes and ketones exhibits a permanent dipole due to the electronegativity difference between the carbon and oxygen atoms.
It’s important to note that the presence of a permanent dipole in a molecule is determined by the combination of these factors. The overall molecular geometry, the electronegativity differences, and the presence of resonance or functional groups all contribute to the formation of a permanent dipole moment.
Understanding the origin of permanent dipoles is essential in explaining molecular properties, intermolecular forces, and the behavior of substances in various chemical and biological processes.
Persistent nature of permanent dipoles
Permanent dipoles are called “permanent” because they persist in molecules even in the absence of an external electric field.
Here are some key points regarding the persistent nature of permanent dipoles:
1. Inherent Molecular Property: Permanent dipoles arise due to the unequal distribution of electrons within a molecule. This unequal distribution is a fundamental characteristic of the molecule itself and remains unchanged unless the molecule undergoes a chemical reaction or experiences a change in its electronic structure.
2. Electron Density Imbalance: The presence of a permanent dipole indicates a consistent imbalance in the electron density distribution within the molecule. This imbalance results in the permanent separation of positive and negative charges, creating the dipole moment. The dipole moment remains constant over time unless there is a change in the molecular structure or bonding.
3. Independent of External Electric Fields: Unlike induced dipoles, which are temporary and dependent on the presence of an external electric field, permanent dipoles persist regardless of the presence or absence of an external field. This means that the magnitude and direction of the permanent dipole moment do not change without an external influence.
4. Contributions to Intermolecular Interactions: Permanent dipoles play a significant role in intermolecular interactions. Dipole-dipole intuitive happens between particles with lasting dipoles, where the positive conclusion of one particle interatomic with the negative conclusion of another atom, driving to appealing strengths. These interactions contribute to various physical and chemical phenomena, such as boiling points, solubilities, and the formation of molecular complexes.
5. Alignment in Electric Fields: Although permanent dipoles are not influenced by external electric fields, they can respond to the presence of such fields. In the presence of an electric field, permanent dipoles tend to align themselves with the field, resulting in additional forces acting on the molecules. This alignment behavior is observed in materials with permanent dipole moments, such as certain crystals and ferroelectric substances.
Understanding the persistent nature of permanent dipoles is crucial in explaining the behavior of molecules, intermolecular forces, and the properties of materials. Permanent dipoles have implications in a wide range of scientific disciplines, including chemistry, physics, materials science, and biology.
Difference Between Induced Dipole and Permanent Dipole
The main difference between induced dipoles and permanent dipoles lies in their origin, nature, and persistence.
Here are the key differences between induced dipoles and permanent dipoles:
Origin:
• Induced Dipole: Induced dipoles are temporary and arise due to the interaction between an external electric field and the electron cloud surrounding an atom or molecule.
• Permanent Dipole: Permanent dipoles exist even in the absence of an external electric field and arise from factors such as polar covalent bonds, molecular asymmetry, or resonance effects.
Formation:
• Induced Dipole: Induced dipoles are formed as a result of the displacement of electron density when an external electric field is applied. The electron cloud is distorted, leading to a temporary separation of charges and the creation of an induced dipole moment.
• Permanent Dipole: Permanent dipoles are formed due to an uneven distribution of electrons within a molecule. This can be caused by factors like polar covalent bonds or molecular asymmetry, resulting in the permanent separation of positive and negative charges and the presence of a permanent dipole moment.
Persistence:
• Induced Dipole: Induced dipoles are temporary. They exist only in the presence of an external electric field. Once the external field is removed, the electron cloud returns to its original configuration and the induced dipole moment disappears.
• Permanent Dipole: Permanent dipoles persist even in the absence of an external electric field. They are inherent properties of molecules and remain unchanged unless there is a change in the molecular structure or bonding.
Interactions:
• Induced Dipole: Induced dipoles play a role in intermolecular forces, particularly London dispersion forces. They arise due to temporary fluctuations in electron density and induce dipoles in neighboring molecules. These temporary interactions contribute to physical properties such as boiling points and molecular interactions.
• Permanent Dipole: Permanent dipoles contribute to intermolecular forces, specifically dipole-dipole interactions. These interactions occur between molecules with permanent dipoles and lead to attractive forces. Permanent dipoles also play a role in other phenomena such as solvation processes and the behavior of polar molecules.
Understanding the difference between induced dipoles and permanent dipoles is essential in explaining various molecular properties, intermolecular interactions, and material behaviors in different scientific fields.
Comparison Chart
Sure! Here’s a comparison chart highlighting the key differences between induced dipoles and permanent dipoles:
| Induced Dipole | Permanent Dipole |
|---|---|
| Arises due to interaction with an external electric field | Arises from factors such as polar covalent bonds, molecular asymmetry, or resonance effects |
| Formed by the displacement of electron density when an external electric field is applied | Formed by an uneven distribution of electrons within a molecule |
| Temporary – disappears when the external electric field is removed | Persistent – exists even in the absence of an external electric field |
| Temporary separation of charges | Permanent separation of charges |
| Contributes to temporary intermolecular forces (London dispersion forces) | Contributes to permanent intermolecular forces (dipole-dipole interactions) |
| Induced dipole-induced dipole interactions, temporary polarization | Permanent dipoles in water (H2O), ammonia (NH3), etc. |
| Reversible – dipole moment changes with the presence or absence of the external electric field | Irreversible – dipole moment remains constant unless there is a change in the molecular structure or bonding |
Please note that this chart provides a general overview, and the specific characteristics of induced dipoles and permanent dipoles can vary depending on the context and molecules involved.
Conclusion
Understanding the difference between induced dipoles and permanent dipoles is crucial in comprehending the intricacies of molecular interactions. Permanent dipoles arise due to differences in electronegativity within a covalent bond, while induced dipoles result from external influences. These dipoles play essential roles in determining various chemical and physical properties of substances. By exploring their characteristics and effects, we gain a deeper understanding of the world at the molecular level.
