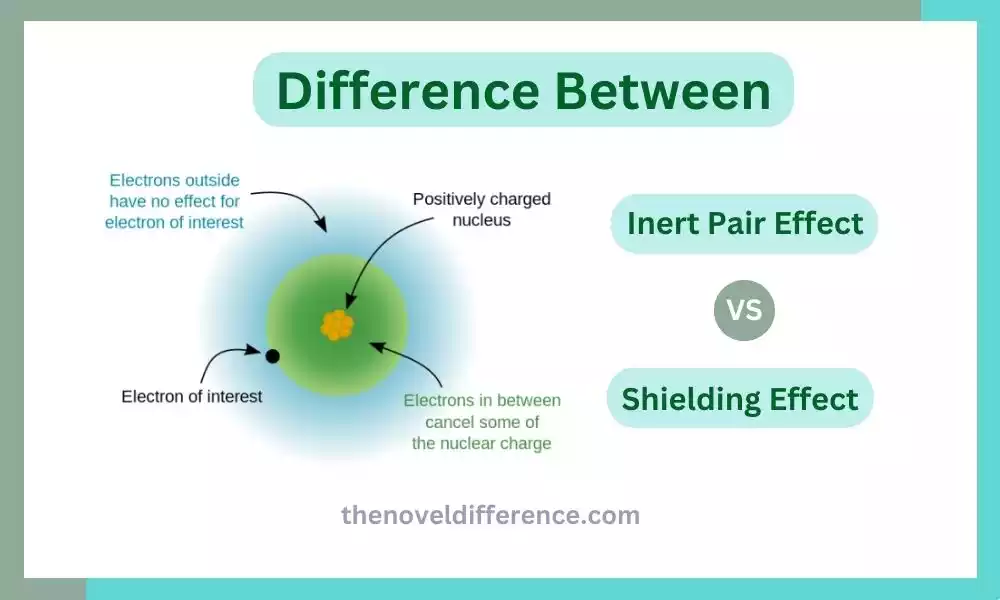
Inert Pair Effect and Shielding Effect: Some numerous concepts and phenomena govern the behavior of elements and their compounds. Two such intriguing concepts are the inert pair effect and the shielding effect. These terms may sound similar, but they refer to distinct phenomena with significant implications for understanding the behavior of elements in various chemical reactions. We will delve deep into the difference between the inert pair effect and the shielding effect, exploring their definitions, characteristics, and practical applications. By the end, you will have a clear understanding of these phenomena and their significance in the world of chemistry.
Importance of understanding these effects in the context of chemistry
Understanding the effects of electron shielding and the inert pair effect is crucial in the context of chemistry for several reasons:
1. Predicting Chemical Reactivity: Electron shielding and the inert pair effect play significant roles in determining the chemical reactivity of elements and compounds. By understanding these effects, chemists can predict the likelihood of certain chemical reactions and design compounds with desired properties. This knowledge is particularly important in fields such as drug discovery, materials science, and catalysis.
2. Explaining Periodic Trends: The periodic table is organized based on patterns and trends in elemental properties. Electron shielding and the inert pair effect contribute to these trends by influencing properties such as atomic radius, ionization energy, and electron affinity. By understanding these effects, scientists can explain and interpret periodic trends, allowing for a deeper understanding of the behavior of elements across the periodic table.
3. Designing Compounds with Desired Properties: Electron shielding and the inert pair effect can impact the stability and reactivity of compounds. Understanding these effects enables chemists to design compounds with specific characteristics, such as stability in certain oxidation states or the ability to undergo specific chemical reactions. This knowledge is vital in fields like materials science, where tailored compounds with desired properties are sought after for various applications.
4. Exploring Unusual Elemental Behavior: The inert pair effect, in particular, leads to unique behavior in certain elements, where lower oxidation states are more stable than expected. This phenomenon has significant implications for the chemistry of elements such as lead, thallium, and bismuth. Understanding the inert pair effect allows scientists to delve into these unusual behaviors, providing insights into the behavior of elements that deviate from typical periodic trends.
5. Advancing Scientific Knowledge: Electron shielding and the inert pair effect are fundamental concepts in chemistry. By studying and understanding these effects, scientists can deepen their understanding of atomic and molecular behavior, contributing to the advancement of scientific knowledge and the development of new theories and models.
Comprehending the effects of electron shielding and the inert pair effect is essential in chemistry for predicting chemical reactivity, explaining periodic trends, designing compounds with desired properties, exploring unusual elemental behavior, and advancing scientific knowledge.
Definition of Inert Pair Effect and Shielding Effect
Inert Pair Effect: The inert pair effect refers to the tendency of certain heavier elements, particularly those in Groups 13 to 16 of the periodic table, to exhibit a preference for lower oxidation states compared to what is expected based on their electron configurations. These elements have valence electrons in the s and p orbitals, and due to the increased stability of the filled d orbitals, they often resist participating in chemical reactions that would involve the loss of these electrons. As a result, they preferentially form compounds in lower oxidation states.
Shielding Effect: The shielding effect, also known as electron shielding or screening, is the phenomenon where the inner electron shells of an atom shield or screen the outer valence electrons from the full attractive force of the positively charged nucleus. This occurs due to repulsion between the negatively charged electrons in the inner shells and the valence electrons. The shielding effect reduces the net attractive force experienced by the valence electrons, making them less tightly held and more easily removed in chemical reactions. Consequently, the shielding effect influences various atomic properties such as atomic radius, ionization energy, and electron affinity.
These two effects, although related to electron behavior in atoms, have distinct characteristics and consequences. The inert pair effect primarily relates to the preference for lower oxidation states in certain heavy elements, while the shielding effect involves the screening of valence electrons by inner electron shells, affecting various atomic properties.
What is Shielding Effect?
The shielding effect, also known as electron shielding or screening, refers to the reduction in the net attractive force experienced by the valence electrons in an atom due to the presence of inner electron shells. It occurs because the inner electron shells partially block or shield the outer valence electrons from the full positive charge of the nucleus.
Key points about the shielding effect include:
- Inner Electron Shells: An atom consists of multiple electron shells or energy levels, with each shell accommodating a specific number of electrons. The inner shells are closer to the nucleus and are occupied by electrons with lower energy levels.
- Electron Repulsion: Electrons are negatively charged particles, and they repel each other due to their like charges. The inner electrons repel the outer valence electrons, creating a repulsive force between them.
- Shielding Effect: The repulsive force between the inner and outer electrons reduces the effective nuclear charge experienced by the valence electrons. The inner electrons act as a shield, decreasing the attraction of the positively charged nucleus toward the outer electrons.
- Effective Nuclear Charge: The effective nuclear charge is the net positive charge experienced by an electron in an atom, taking into account the shielding effect. It is given by the difference between the actual nuclear charge and the shielding constant.
- Impact on Atomic Properties: The shielding effect influences various atomic properties, including atomic radius, ionization energy, and electron affinity. It plays a role in determining the size of an atom, as the repulsion between electrons pushes the outer electrons farther from the nucleus, increasing the atomic radius.
- Ionization Energy: The shielding effect reduces the ionization energy, which is the energy required to remove an electron from an atom. The outer valence electrons experience less attraction from the nucleus, making them easier to remove.
- Electron Affinity: The shielding effect can also affect electron affinity, which is the energy change associated with adding an electron to an atom. The presence of inner electrons can partially shield the added electron from the nucleus, reducing the electron affinity.

The shielding effect is an important concept in understanding atomic structure and the behavior of electrons in atoms. It helps explain trends in atomic properties across the periodic table and provides insights into chemical reactivity and the formation of chemical bonds.
Atomic radius
Atomic radius refers to the size of an atom, specifically the distance between the nucleus of an atom and the outermost edge of its electron cloud. It is a fundamental property of an atom that directly affects its chemical and physical behavior.
The atomic radius is typically measured as the distance between the nuclei of two adjacent atoms in a solid or a molecule. Since the electron cloud surrounding an atom does not have a well-defined boundary, determining the exact atomic radius is somewhat challenging. Atomic radius is usually estimated by considering various factors and using empirical data.
Several trends and factors influence the atomic radius:
1. Periodic Trend: As one moves across a period (horizontal row) in the periodic table from left to right, the atomic radius generally decreases. This is due to an increase in the effective nuclear charge, which pulls the outermost electrons closer to the nucleus, resulting in a smaller atomic size.
2. Group Trend: Moving down a group (vertical column) in the periodic table, the atomic radius generally increases. This is primarily because new electron shells are added as you move down, increasing the distance between the nucleus and the outermost electrons.
3. Electron Shielding: Electron shielding plays a significant role in determining atomic radius. The presence of inner electron shells shields the outermost electrons from the full attractive force of the nucleus, resulting in a larger atomic radius.
4. Ionic Radius: The atomic radius can change depending on whether an atom loses or gains electrons to form an ion. Cations (positively charged ions) are typically smaller than their parent atoms, as they lose one or more outermost electrons. Anions (negatively charged ions) are generally larger than their parent atoms, as they gain electrons and experience increased electron-electron repulsion.
Atomic radius is an important factor in understanding chemical bonding, reactivity, and the physical properties of elements. It affects the strength of chemical bonds, such as covalent and metallic bonds, and influences properties like melting and boiling points, solubility, and conductivity. By analyzing atomic radius trends, scientists can make predictions about an element’s behavior and its interactions with other elements in chemical reactions.
Ionization energy
Ionization energy, also known as ionization potential, refers to the amount of energy required to remove an electron from a neutral atom or ion in its gaseous state. It is a measure of the strength of the bond between an electron and the nucleus of an atom.
When an atom gains or loses electrons, it forms an ion. The ionization energy specifically refers to the process of removing an electron from a neutral atom to form a positively charged ion (cation). The energy required to remove subsequent electrons from the same atom increases progressively.
Key points regarding ionization energy include:
1. First Ionization Energy: This is the energy required to remove the outermost or valence electron from an atom in its neutral state to form a singly charged positive ion (cation). The first ionization energy is generally lower for metals compared to nonmetals because metals have loosely held valence electrons.
2. Second and Higher Ionization Energies: After the first electron is removed, subsequent ionization energies increase significantly. It becomes increasingly difficult to remove additional electrons since the remaining electrons experience a greater effective nuclear charge and reduced electron-electron repulsion.
3. Periodic Trend: Ionization energy generally increases from left to right across a period in the periodic table. This is due to the increasing effective nuclear charge as the number of protons in the nucleus increases, which attracts the electrons more strongly, requiring more energy for their removal.
4. Group Trend: Ionization energy generally decreases from top to bottom within a group in the periodic table. This is primarily because the outermost electrons are located in higher energy levels and are shielded by inner electron shells, reducing the effective nuclear charge they experience.
5. Factors Affecting Ionization Energy: The ionization energy is influenced by several factors, including atomic radius (larger atoms have lower ionization energy), effective nuclear charge (higher effective nuclear charge leads to higher ionization energy), and electron configuration (filled or half-filled subshells have higher ionization energy due to increased stability).
Ionization energy is an important concept in understanding chemical reactivity, as it determines an element’s ability to form positive ions and participate in chemical reactions. It is used to explain trends in the periodic table, predict the behavior of elements, and understand the stability of ions and compounds.
What is the Inert Pair Effect?
The inert pair effect is a phenomenon observed in certain heavier elements, particularly those in Groups 13 to 16 of the periodic table. It refers to the tendency of these elements to exhibit a preference for lower oxidation states compared to what is expected based on their electron configurations.
Electrons are arranged in energy levels or electron shells. The outermost shell, known as the valence shell, contains the valence electrons that participate in chemical bonding. The inert pair effect specifically relates to the behavior of valence electrons in the s and p orbitals.
Elements affected by the inert pair effect have valence electrons in the s and p orbitals that are closer to the nucleus and experience a stronger attractive force from the positively charged nucleus. As a result, these electrons are less likely to participate in chemical reactions that involve the loss of these electrons.
The inert pair effect arises due to two main factors:
1. Increasing Stability of Filled d Orbitals: The filled d orbitals provide additional stability compared to the s and p orbitals. As a result, the d orbitals are less likely to participate in bonding or chemical reactions. The preference for lower oxidation states allows the element to retain its valence electrons in the s and p orbitals, rather than losing them to achieve a higher oxidation state.
2. Poor Overlap and Bonding: The s and p orbitals involved in the inert pair effect have less effective overlap with other orbitals, making it more difficult for these valence electrons to participate in covalent bonding. This leads to a higher likelihood of forming compounds in lower oxidation states where the s and p orbitals are not involved in bonding.
Consequences of the inert pair effect include:
1. Lower Oxidation States: Elements affected by the inert pair effect tend to exhibit lower oxidation states in compounds. For example, lead (Pb) typically forms compounds in the +2 oxidation state instead of the +4 oxidation state expected based on its electron configuration.
2. Stability of Higher Oxidation States: Elements prone to the inert pair effect often exhibit stability in their lower oxidation states, while higher oxidation states are less stable. This is because the loss of valence electrons from the s and p orbitals becomes energetically unfavorable due to increased electron-nuclear attraction.
3. Chemical Reactivity Differences: The inert pair effect leads to significant differences in the chemical reactivity of elements. Elements prone to the inert pair effect are often less reactive and less likely to undergo chemical reactions that involve the loss of valence electrons.
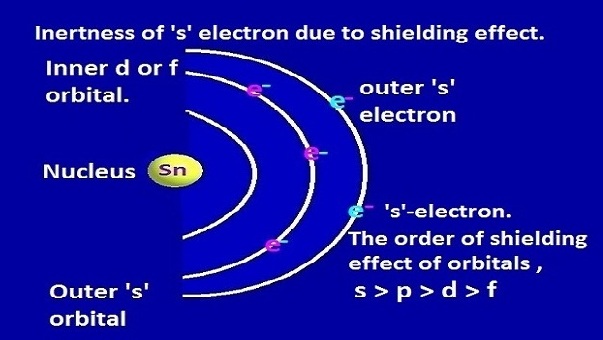
The inert pair effect highlights the unique behavior of certain heavy elements in terms of their preference for lower oxidation states and limited participation of valence electrons in bonding. Understanding this effect is important in predicting the chemical behavior of elements and designing compounds with desired properties.
Lower oxidation states
Lower oxidation states refer to the states of lower electron loss or higher electron gain that an atom can exhibit in chemical compounds. It signifies the number of electrons an atom loses or gains when it undergoes oxidation or reduction, respectively, in a chemical reaction.
Elements affected by this phenomenon tend to prefer lower oxidation states despite having the potential for higher oxidation states based on their electron configurations. The inert pair effect is commonly observed in heavier elements, especially those in Groups 13 to 16 of the periodic table.
Here are a few examples of elements that exhibit lower oxidation states due to the inert pair effect:
1. Lead (Pb): The inert pair effect is prominent in lead. Based on its electron configuration, lead could potentially achieve a +4 oxidation state by losing all its valence electrons. However, in many compounds, lead typically exhibits a +2 oxidation state due to the retention of its valence s and p electrons.
2. Thallium (Tl): Thallium exhibits the inert pair effect and shows a preference for the +1 oxidation state instead of the +3 oxidation state expected based on its electron configuration.
3. Bismuth (Bi): Bismuth is another element affected by the inert pair effect. While it could theoretically achieve a +5 oxidation state, it predominantly forms compounds in the +3 oxidation state.
The retention of the valence s and p electrons contribute to the lower oxidation states observed. The inert pair effect arises due to the increased stability associated with filled d orbitals in these heavy elements, making the loss of valence electrons less favorable.
The tendency to exhibit lower oxidation states in elements affected by the inert pair effect has important implications for their chemical reactivity and the types of compounds they form. It impacts the stability and behavior of these elements in various chemical reactions and contributes to the unique characteristics of compounds containing such elements.
Stability of higher oxidation states
The stability of higher oxidation states refers to the tendency of certain elements to exhibit more stability and resistance to undergoing oxidation to higher valence states. This stability is often observed in elements affected by the inert pair effect, particularly in heavier elements in Groups 13 to 16 of the periodic table.
The valence electrons in the s and p orbitals are closer to the nucleus and experience a stronger attractive force. As a result, these elements exhibit a preference for lower oxidation states, where the s and p electrons are retained rather than lost.
The stability of higher oxidation states in elements affected by the inert pair effect can be attributed to several factors:
1. Increasing Effective Nuclear Charge: As electrons are lost and the oxidation state increases, the effective nuclear charge experienced by the remaining electrons increases. This increased attraction between the nucleus and the remaining valence electrons makes it energetically unfavorable to further oxidize the element.
2. Poor Overlap and Bonding: The s and p orbitals involved in the inert pair effect have limited overlap with other orbitals, leading to difficulties in forming covalent bonds. This reduces the tendency of the element to participate in oxidation reactions that involve the loss of valence electrons.
3. Increased Stability of Lower Oxidation States: Lower oxidation states are more stable for elements experiencing the inert pair effect due to the retention of valence electrons in the s and p orbitals. The stability associated with having filled or half-filled orbitals contributes to the resistance of these elements to undergo further oxidation.
The stability of higher oxidation states in elements affected by the inert pair effect has significant implications for their chemical behavior. It often results in a restricted range of oxidation states exhibited by these elements, with lower oxidation states being more prevalent. This behavior is observed in elements such as lead (Pb), thallium (Tl), and bismuth (Bi), which preferentially form compounds in lower oxidation states despite having the potential for higher oxidation states based on their electron configurations.
Understanding the stability of higher oxidation states in elements affected by the inert pair effect is crucial for predicting their reactivity, designing compounds, and explaining the unique behavior of these elements within the periodic table.
Difference Between the Inert Pair Effect and Shielding Effect
The Inert Pair Effect and Shielding Effect are two distinct phenomena that occur in chemistry and have different implications for the behavior of atoms and their electrons.
Here are the key differences between the inert pair effect and the shielding effect:
1. Definition and Nature:
• Inert Pair Effect: The inert pair effect refers to the tendency of certain heavier elements, particularly those in Groups 13 to 16 of the periodic table, to exhibit a preference for lower oxidation states compared to what is expected based on their electron configurations. It is related to the resistance of valence electrons in the s and p orbitals to participate in chemical reactions involving electron loss.
• Shielding Effect: The shielding effect, also known as electron shielding or screening, occurs when inner electron shells partially block or shield the outer valence electrons from the full attractive force of the positively charged nucleus. It arises from the repulsion between negatively charged electrons and affects various atomic properties.
2. Electron Behavior:
• Inert Pair Effect: The inert pair effect primarily focuses on the behavior of valence electrons in the s and p orbitals of certain heavy elements. These electrons are less likely to participate in chemical reactions that involve their loss, leading to a preference for lower oxidation states.
• Shielding Effect: The shielding effect relates to the interaction between inner and outer electrons in an atom. The inner electron shells shield or screen the outer valence electrons from the full positive charge of the nucleus, reducing the net attractive force experienced by the valence electrons.
3. Factors Involved:
• Inert Pair Effect: The inert pair effect is influenced by the increasing stability associated with filled d orbitals in heavy elements, making the loss of valence electrons less favorable. It also arises from poor orbital overlap and bonding of the s and p electrons.
• Shielding Effect: The shielding effect depends on the number of electrons present in the inner shells, as well as the distance between the valence electrons and the nucleus. More occupied inner shells and greater distances lead to stronger shielding.
4. Consequences:
• Inert Pair Effect: The inert pair effect results in heavy elements exhibiting a preference for lower oxidation states, leading to different chemical reactivity and compound formation patterns compared to what would be expected based on their electron configurations.
• Shielding Effect: The shielding effect influences various atomic properties such as atomic radius, ionization energy, and electron affinity. It affects the strength of the attraction between the nucleus and valence electrons, leading to changes in the size of the atom and the ease of removing or adding electrons.
The inert pair effect relates to the preference for lower oxidation states in certain heavy elements, specifically involving the behavior of valence electrons. The shielding effect refers to the screening of valence electrons by inner electron shells, affecting various atomic properties. Although both effects involve electron behavior, they are distinct in terms of their definitions, mechanisms, and consequences.
Comparison Chart
Here is a comparison chart highlighting the key differences between the Inert Pair Effect and Shielding Effect:
| Inert Pair Effect | Shielding Effect |
|---|---|
| Preference for lower oxidation states in certain heavy elements due to the resistance of valence electrons in the s and p orbitals to participate in chemical reactions involving electron loss. | Partial blocking or shielding of outer valence electrons by inner electron shells, reduces the net attractive force experienced by the valence electrons. |
| Focuses on the behavior of valence electrons in the s and p orbitals of certain heavy elements. | Relates to the interaction between inner and outer electrons in an atom. |
| Influenced by the increasing stability associated with filled d orbitals in heavy elements and poor orbital overlap and bonding of the s and p electrons. | Depends on the number of electrons present in the inner shells and the distance between the valence electrons and the nucleus. |
| Leads to heavy elements exhibiting a preference for lower oxidation states, impacting their chemical reactivity and compound formation patterns. | Affects various atomic properties such as atomic radius, ionization energy, and electron affinity, influencing the size of the atom and the ease of removing or adding electrons. |
It’s important to note that while these two effects have distinct characteristics, they can both play a significant role in understanding the behavior and properties of atoms and elements in chemistry.
Conclusion
The Inert Pair Effect and Shielding Effect are two distinct phenomena with their own set of characteristics and implications in chemistry. The inert pair effect relates to the reluctance of s-electrons to participate in bonding in heavy elements, while the shielding effect involves the shielding of outer electrons from the full attraction of the nucleus by inner electrons. Understanding these effects is vital in unraveling the mysteries of periodic trends, oxidation states, and chemical behavior of elements. By exploring the intricacies of the Inert Pair Effect and Shielding Effect, we gain valuable insights into the fascinating world of chemistry.