Importance of Ion exchange and Reverse osmosis
Ion exchange and reverse osmosis play crucial roles in ensuring access to clean, safe, and high-quality water, albeit through different mechanisms.
Ion exchange is valuable for selectively removing specific ions and contaminants from water by exchanging them with ions in a resin. This process is significant in applications where targeted removal of particular minerals, heavy metals, or specific ions is necessary, such as in industrial settings, water softening for households, or in purifying drinking water.
Reverse osmosis, on the other hand, operates by forcing water through a semi-permeable membrane to eliminate a wide range of impurities, including bacteria, viruses, chemicals, and dissolved solids. It’s a versatile and effective method widely used in households, commercial systems, and industries to produce purified drinking water and to desalinate seawater.
Both ion exchange and reverse osmosis contribute significantly to ensuring water purity, catering to various needs ranging from residential drinking water to industrial processes, enhancing health and overall quality of life.
What is Ion Exchange?
Ion exchange is a water treatment process that involves the exchange of ions between a solid material, known as an ion exchange resin, and the water being treated. The resin is typically in the form of small beads or granules, and it is designed to attract and hold ions with the opposite charge.
The process begins as water flows through a bed of ion exchange resin. The resin is coated with charged ions, which can be either positively charged (cation exchange) or negatively charged (anion exchange). As the water passes through the resin bed, the ions in the water are attracted to the charged sites on the resin and are exchanged with ions of the same charge. This results in the removal of certain ions from the water and their replacement with different ions.
Ion exchange is commonly used for various purposes in water treatment. It Can Be Employed to Remove Dissolved Minerals, Such as Calcium, Magnesium, and Iron, Which Cause Water Hardness. It is Also Effective in Removing Heavy Metals, Such as Lead, Mercury, and Arsenic, From Water. Ion Exchange can be used for the Removal of Specific Contaminants, Such as Nitrates, Sulfates, and Organic Compounds.
Once the resin becomes saturated with the exchanged ions, it needs to be regenerated or recharged to restore its ion exchange capacity. This is typically done by passing a regenerating solution, such as salt brine for cation exchange or acid or base solution for anion exchange, through the resin bed. The regenerating solution displaces the unwanted ions from the resin, allowing it to be reused for further ion exchange.
Ion exchange is a versatile and effective water treatment method for removing specific ions and contaminants, improving water quality, and addressing various water treatment needs.
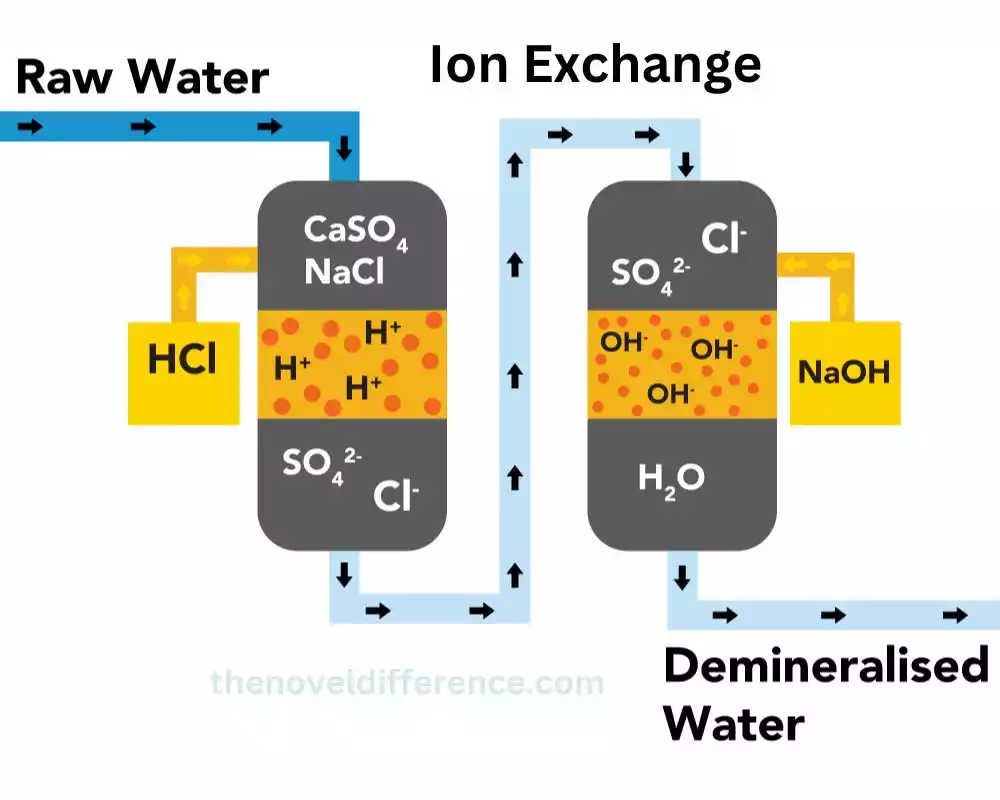
How ion exchange works
Here’s a simplified explanation of how ion exchange works:
- Ion exchange resins: These are small beads or granules packed into a container. They contain charged sites that are attractions.
- Ion swapping: When water passes through the resin bed, ions in the water stick to the charged sites on the resin, swapping places with ions already on the resin.
- Removal of impurities: Undesirable ions in the water, like calcium, magnesium, or heavy metals, get captured by the resin while harmless ions, like sodium, are released.
- Regeneration: Over time, the resin becomes saturated with captured ions. To reuse the resin, it undergoes a regeneration process using a salt solution (brine) to flush out the captured ions, restoring the resin’s ability to exchange ions again.
- Continuous cycle: The process continues in a cyclical manner, allowing the resin to repeatedly capture ions from the water and release other ions, effectively purifying the water as it passes through the ion exchange system.
Ion exchange involves the exchange of unwanted ions in the water for more acceptable ions in the resin, thus purifying the water by removing specific contaminants or minerals.
Advantages and disadvantages of ion exchange
Advantages of ion exchange:
- Selective Removal: Targets specific ions or contaminants, allowing tailored purification.
- Effective for Softening: Removes hardness-causing minerals like calcium and magnesium.
- Reusable Resins: Resins can be regenerated and reused multiple times.
- Fast Process: Provides rapid purification of water.
Disadvantages of ion exchange:
- Limited Contaminant Range: Not effective against all types of contaminants.
- High Maintenance: Regular regeneration and resin replacement are required.
- Waste Generation: The regeneration process produces salty wastewater.
- Expense: Initial setup costs and operational expenses can be high.
What is Reverse Osmosis?
Reverse Osmosis is a Water Purification Process That uses a Semi-Permeable Membrane to Remove a Wide Range of Contaminants From Water. It Operates Based on the Principle of Applying Pressure to the Water to Force It Through the Membrane While Blocking the Passage of Dissolved Solids, Salts, and Other impurities.
Here’s how reverse osmosis works:
1. Pressure Application: Reverse osmosis requires the application of external pressure to overcome the natural osmotic pressure and drive the water through the membrane. This pressure can be generated using a pump or other pressurization methods.
2. Semi-Permeable Membrane: The heart of the reverse osmosis system is the semi-permeable membrane. It consists of a thin, dense barrier that allows water molecules to pass through while rejecting the majority of dissolved salts, minerals, bacteria, viruses, and other impurities present in the water. The membrane’s microscopic pores or channels are small enough to prevent the passage of most contaminants, ensuring high-quality water output.
3. Separation of Contaminants: As the pressurized water flows across the membrane surface, the contaminants in the water are retained by the membrane, forming a concentrated solution called the reject stream or brine. Simultaneously, the purified water, referred to as permeate, passes through the membrane and is collected for use or storage.
4. Reject Stream Disposal: The reject stream, containing the concentrated contaminants, is typically discharged from the system. However, some advanced reverse osmosis systems incorporate technologies to improve efficiency and reduce water waste by recycling or reusing the reject stream.
5. Post-Treatment and Storage: The permeate, which has passed through the reverse osmosis membrane, undergoes further post-treatment, such as remineralization and disinfection, to enhance taste, quality, and stability. It is then stored in a tank for future use, such as drinking water or other applications.
Reverse Osmosis is Widely used for Various Purposes, Including Residential Drinking Water Systems, Industrial Water Purification, Desalination of Seawater, and Wastewater Treatment. It is a Highly Effective Method for Producing Clean, Purified Water by Removing Dissolved Solids, Salts, Bacteria, Viruses, and Other Impurities, Making It Suitable for a Range of Water Treatment Needs.
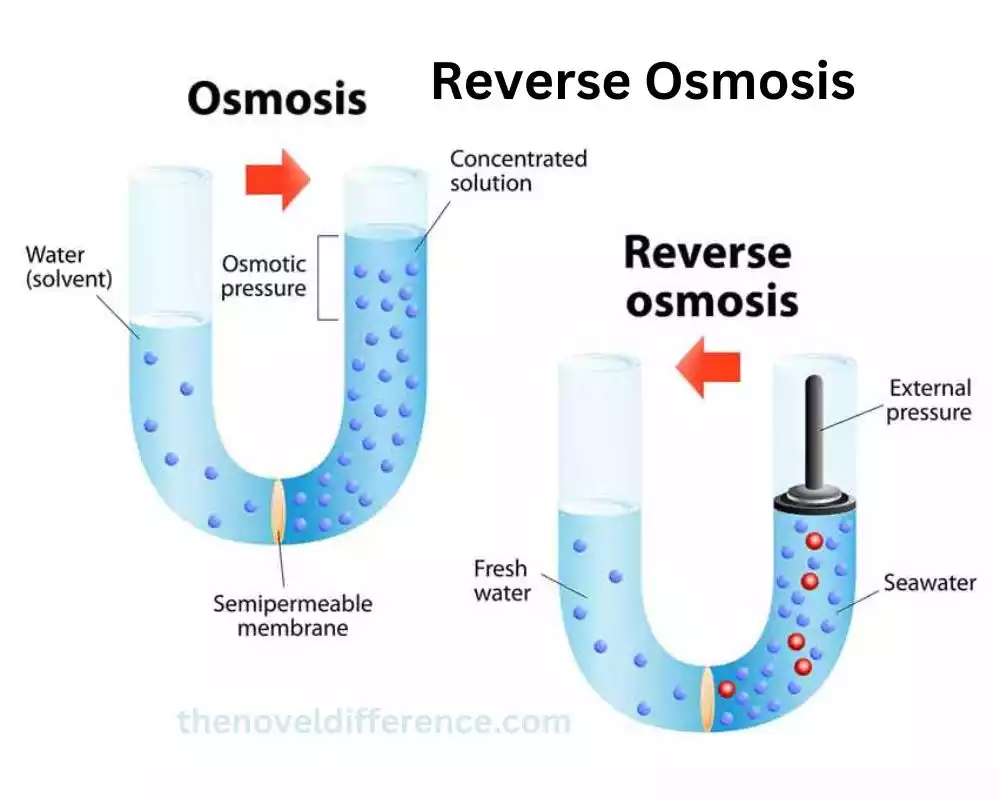
How reverse osmosis works
Reverse Osmosis (RO) Is a Water Purification Process That Works by using Pressure to Force Water Through a Semi-Permeable Membrane, Separating Contaminants From the Water.
Here is a step-by-step explanation of how reverse osmosis works:
1. Pre-filtration: Before entering the reverse osmosis system, the feed water usually goes through pre-filtration to remove larger particles, sediment, and chlorine, which could damage the RO membrane.
2. Pressurization: The feed water is then pressurized using a pump or other means to overcome the osmotic pressure and facilitate the water flow through the RO system. The pressure applied depends on the concentration of contaminants in the feed water and the desired water quality.
3. Semi-permeable Membrane: The pressurized water enters the reverse osmosis system, which consists of a semi-permeable membrane. The membrane contains extremely tiny pores or channels that allow only water molecules to pass through while blocking the majority of dissolved solids, salts, bacteria, viruses, and other contaminants.
4. Contaminant Separation: As the pressurized water passes through the membrane, the contaminants in the water are rejected and left behind. The membrane acts as a physical barrier, selectively allowing the passage of water molecules while effectively removing various impurities. The rejected contaminants are concentrated in what is known as the concentrate or brine stream.
5. Permeate Collection: The purified water that successfully passes through the membrane is known as permeate or product water. It is collected and typically stored in a separate tank for use or distribution. The quality of the permeate depends on the effectiveness of the reverse osmosis system and the specific membrane used.
6. Concentrate Disposal: The concentrate or brine stream, containing the concentrated contaminants, is typically discharged from the system. In some cases, advanced RO systems employ technologies to recover and recycle a portion of the concentrate, reducing water waste and increasing overall efficiency.
7. Post-Treatment: Depending on the desired water quality and specific applications, the permeate from the reverse osmosis system may undergo post-treatment processes such as remineralization, pH adjustment, or disinfection to improve taste, quality, and stability before it is used or consumed.
Reverse Osmosis is Widely used for Drinking Water Purification, Industrial Water Treatment, Desalination of Seawater, and Other Applications Where High-Quality Water is Required. Its ability to remove a wide range of contaminants makes it an effective and popular method for producing clean and purified water.
Advantages and disadvantages of reverse osmosis
Advantages of Reverse Osmosis:
1. Effective Contaminant Removal: Reverse Osmosis is Highly Effective in Removing a Wide Range of Contaminants From Water, Including Dissolved Solids, Salts, Bacteria, Viruses, Heavy Metals, and Certain Organic Compounds. It provides a high level of purification and produces clean, safe drinking water.
2. Versatility: Reverse osmosis can be applied to various water sources, including tap water, well water, brackish water, and seawater. It is a versatile water treatment method suitable for both residential and industrial applications.
3. Improves Taste and Odor: Reverse osmosis helps to improve the taste, odor, and overall quality of water by removing impurities, such as chlorine, that can affect the flavor and smell of the water.
4. Compact and Space-Efficient: Reverse osmosis systems are typically compact and require less space compared to other water treatment technologies. They can be easily installed under sinks or in confined spaces, making them suitable for residential use.
5. Low Energy Consumption: Although reverse osmosis requires energy to operate the pumps, the energy consumption is relatively low compared to other desalination methods. Advances in technology have led to more energy-efficient reverse osmosis systems.
Disadvantages of Reverse Osmosis:
1. Water Waste: Reverse osmosis systems generate a significant amount of wastewater during the purification process. For every gallon of purified water produced, a certain amount of water is discharged as the concentrate or brine stream. This can be a concern in areas with water scarcity.
2. Removal of Beneficial Minerals: Reverse osmosis removes not only harmful contaminants but also beneficial minerals present in water, such as calcium and magnesium. The demineralized water may require remineralization or supplementation to restore essential minerals for optimal health.
3. Slow Process: Reverse osmosis systems have a relatively slow water production rate. The water flow rate is determined by the membrane size and system design. For larger water demands, additional storage tanks or booster pumps may be required.
4. Maintenance and Membrane Replacement: Reverse osmosis systems require regular maintenance, including periodic membrane cleaning and replacement. Fouling and scaling on the membrane can affect system performance and efficiency.
5. Energy Requirement: Reverse osmosis systems rely on pumps to generate the required pressure for water purification. This energy consumption adds to the operational cost of the system.
It is important to consider the specific water quality needs, water source, wastewater management, and maintenance requirements when evaluating the suitability of reverse osmosis for a particular application.
Differences Between Ion Exchange and Reverse Osmosis
Ion Exchange and Reverse Osmosis are two distinct water treatment processes that differ in their mechanisms and applications.
Here are the key differences between Ion Exchange and Reverse Osmosis:
1. Principle:
• Ion Exchange: Ion exchange involves the exchange of ions between a solid resin material and the water being treated. It uses a resin with charged sites to attract and hold ions, resulting in the removal of specific contaminants and the release of other ions into the treated water.
• Reverse Osmosis: Reverse osmosis works by applying pressure to force water through a semi-permeable membrane. The membrane selectively allows water molecules to pass through while blocking dissolved solids, salts, and other impurities, resulting in the separation and removal of contaminants.
2. Contaminant Removal:
• Ion Exchange: Ion exchange is effective for removing specific ions and targeted contaminants from water. It can remove dissolved minerals, heavy metals, and other charged particles based on the resin’s selectivity.
• Reverse Osmosis: Reverse osmosis is highly effective in removing a wide range of contaminants, including dissolved solids, salts, bacteria, viruses, heavy metals, and certain organic compounds. It provides comprehensive purification by blocking contaminants based on size and charge.
3. Selectivity:
• Ion Exchange: Ion exchange can be selective, allowing the removal of specific ions or contaminants based on the type of resin used. It can be tailored to target specific contaminants.
• Reverse Osmosis: Reverse osmosis is non-selective and removes a broad range of contaminants based on their size and charge. It provides comprehensive purification without specific targeting.
4. Maintenance and Regeneration:
• Ion Exchange: Ion exchange resins become saturated over time and require periodic regeneration or replacement. Regeneration involves passing a regenerating solution through the resin bed to restore its ion exchange capacity.
• Reverse Osmosis: Reverse osmosis membranes require regular maintenance, including cleaning and periodic replacement. Fouling, scaling, and other factors can affect membrane performance and longevity.
5. Water Efficiency:
• Ion Exchange: Ion exchange does not inherently wastewater during the treatment process. However, the regeneration process can generate a waste stream that needs proper disposal or treatment.
• Reverse Osmosis: Reverse osmosis systems generate a significant amount of wastewater (concentrate or brine) during the purification process. The water recovery rate depends on system design and can vary.
6. Applications:
• Ion Exchange: Ion exchange is commonly used for water softening to remove hardness-causing minerals, as well as for specific contaminant removals, such as heavy metals, nitrates, and sulfates. It is also used for deionization and demineralization processes.
• Reverse Osmosis: Reverse osmosis is widely used for residential drinking water systems, industrial water treatment, desalination of seawater, and other applications requiring comprehensive water purification.
Choosing between ion exchange and reverse osmosis depends on specific water treatment needs, the type of contaminants present, desired water quality, wastewater management considerations, and system efficiency requirements.
Choosing the Right Method
Choosing the right water treatment method, whether it’s ion exchange or reverse osmosis, depends on various factors and considerations.
Here are some key points to help in the decision-making process:
1. Water Quality Analysis: Begin by conducting a comprehensive water quality analysis to identify the specific contaminants present in the water. This analysis will help determine which method is more suitable for effectively addressing those contaminants.
2. Targeted Contaminant Removal: Consider the specific contaminants that need to be removed. Ion exchange is effective for the targeted removal of specific ions or contaminants, such as hardness-causing minerals or heavy metals. Reverse osmosis, Provides comprehensive removal of a wide range of contaminants, including dissolved solids, salts, bacteria, and viruses.
3. Water Usage and Application: Consider the intended use and application of the treated water. For example, if the primary concern is water softening to reduce hardness, ion exchange may be more appropriate. If the goal is to achieve high-quality drinking water or industrial-grade water purification, reverse osmosis may be the preferred choice.
4. Water Efficiency and Waste Management: Assess the water efficiency and wastewater management considerations. Ion exchange does not inherently wastewater during treatment, while reverse osmosis systems generate a significant amount of wastewater (concentrate or brine). Consider the availability of water resources and the environmental impact of wastewater disposal.
5. System Maintenance and Operation: Evaluate the maintenance requirements and operational considerations of each method. Ion exchange systems require resin regeneration or replacement, while reverse osmosis systems need periodic membrane cleaning and potential replacement. Consider the availability of maintenance resources and the associated costs.
6. Cost Considerations: Assess the initial investment cost, operational costs, and lifetime cost of each method. Consider factors such as equipment, maintenance, energy consumption, and consumables (e.g., resin, membranes). Compare these costs against the desired water quality and treatment objectives.
7. Expert Consultation: Consult with water treatment professionals or experts who can provide guidance based on their expertise and experience. They can help assess the specific water treatment requirements and recommend the most appropriate method based on the given circumstances.
The choice between ion exchange and reverse osmosis depends on the specific water treatment needs, the type and concentration of contaminants, desired water quality goals, wastewater management considerations, and available resources. It may also be beneficial to consider the potential for combining or integrating both methods in certain cases to achieve optimal results.
Conclusion
Understanding the differences between ion exchange and reverse osmosis is crucial for selecting the most suitable water treatment method for your specific needs. Ion exchange excels in removing specific ions and is often used for water softening and targeted contaminant removal. Reverse osmosis offers comprehensive filtration and is ideal for producing high-purity water. Consider factors such as water quality, cost, efficiency, and maintenance requirements when making your decision. Consulting a water treatment professional can provide valuable guidance in choosing the optimal solution. Remember, clean and safe water is essential for a healthy life!
