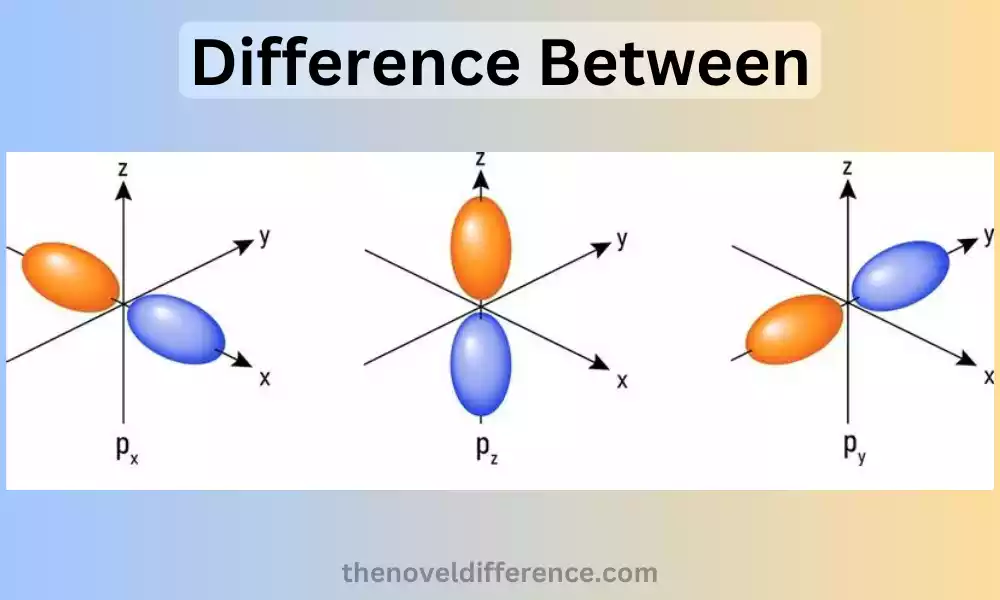
Introduction to Px Py and Pz Orbitals
The Px, Py, and Pz orbitals are a set of three p orbitals that belong to the second energy level (n = 2) of an atom. These orbitals have specific shapes and orientations, and they play a crucial role in understanding molecular bonding and the geometry of molecules.
Px orbital:
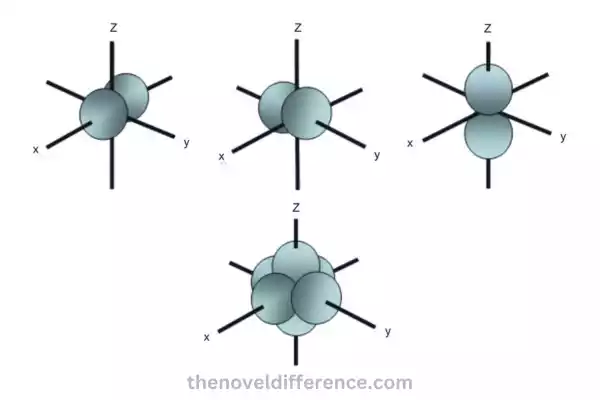
The Px orbital is one of the three p orbitals and is oriented along the x-axis. This object resembles a dumbbell with a node at its nucleus and two lobes on either side extending along its x-axis. The lobes are of equal size and have opposite phases, indicating the opposite signs of the wave function.
Py orbital:
The Py orbital is another p orbital and is oriented along the y-axis. Similar to the Px orbital, it has a dumbbell shape with lobes on either side of the nucleus along the y-axis. The lobes are also of equal size but are perpendicular to the lobes of the Px orbital. The Py orbital also has opposite phases in its lobes.
Pz orbital:
The Pz orbital is the third p orbital and is oriented along the z-axis. It has a dumbbell shape, similar to the Px and Py orbitals, but the lobes are oriented along the z-axis. The lobes are again of equal size and have opposite phases.
The Px, Py, and Pz orbitals are mutually perpendicular to one another; their axes are aligned along the x, y, and z axes respectively. This arrangement allows for the complete coverage of space around the nucleus, providing a description of the electron density in three dimensions.
These p orbitals are involved in various chemical processes, including molecular bonding, hybridization, and the formation of double and triple bonds. The orientation and interaction of these orbitals determine the shape and geometry of molecules, influencing their properties and reactivity.
Understanding the differences between Px, Py, and Pz orbitals is essential for comprehending molecular orbitals, molecular shapes, and the behavior of electrons in chemical reactions.
What are Atomic Orbitals?
Atomic orbitals are regions of space around an atomic nucleus where electrons are most likely to be found. They are fundamental to understanding the behavior and properties of atoms.
Quantum mechanics uses wave functions to model electrons within an atom’s composition; these mathematical functions determine the probability that an electron could exist somewhere within space at any one moment in time.
Atomic orbitals can be identified using three quantum numbers, known as principal quantum number (n), azimuthal quantum number (l), and magnetic quantum number (m). These quantum numbers define the energy, shape, and orientation of the orbitals.
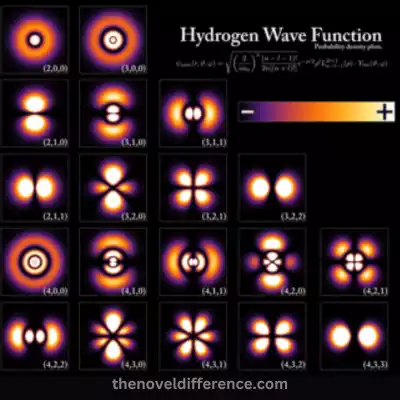
The principal quantum number (n) determines the energy level or shell of the orbital. It can take on positive integer values starting from 1 (n = 1) and represents the distance of the orbital from the nucleus. Orbitals with higher values of n are farther from the nucleus and have higher energy.
The azimuthal quantum number (l) determines the shape of the orbital and the angular momentum of the electron. It can take on values from 0 to (n-1). The value l corresponds with different subshells; an orbital of type S represents subshell 0 while orbitals with values 1 or 2 represent different orbitals in different subshells; for instance, l = 0 represents an S orbital, 1 represents P orbitals, and so forth.
The magnetic quantum number (m) determines the orientation of the orbital in space. It can take on integer values ranging from -l to +l, including zero. The number of allowed values for m depends on the value of l.
The combination of these quantum numbers determines the unique characteristics of each atomic orbital. Examples of hydrogen orbitals include one (n equals 1) that serves as one of its orbitals; in contrast, its second shell (n equals 2) contains three orbitals with different energy states such as an S orbital, and three P orbitals px, py and pz; its third (n equals 3) is composed of one S orbital with three P orbitals as well as five additional orbitals and so forth.
Atomic orbitals provide the foundation for understanding electron configurations, chemical bonding, and the behavior of atoms in various chemical reactions and processes.
Importance of understanding the differences between Px, Py, and Pz orbitals
Understanding the distinctions among Px, Py, and Pz orbitals is essential for a number of reasons:
Molecular Geometry: Px, Py, and Pz orbitals play an essential part in defining the three-dimensional shape and geometry of molecules. By understanding the orientations of these orbitals, we can predict the bond angles and molecular arrangements in chemical compounds. This knowledge is essential in fields such as organic chemistry, where molecular shape impacts the reactivity and properties of compounds.
Bonding and Hybridization: The Px, Py, and Pz orbitals are involved in molecular bonding and hybridization. When atoms join to create compounds, the orbital of their molecules can interact with those of other nearby atoms and form covalent bonds between the atoms. Understanding the differences in orientation and spatial distribution of these orbitals helps explain the types of bonds formed (e.g., sigma or pi bonds) and the resulting molecular structure.
Electron Configuration: The Px, Py, and Pz orbitals contribute to the electron configuration of atoms and molecules. They provide insight into the distribution and arrangement of electrons in different energy levels and subshells. By understanding the differences between these orbitals, we can determine electron occupancy and predict the chemical behavior of elements and compounds.
Spectroscopy and Excited States: Spectroscopic techniques, such as electron spectroscopy and molecular orbital theory, rely on the understanding of orbital differences. The transition of electrons between Px, Py, and Pz orbitals can result in the absorption or emission of specific wavelengths of light. This knowledge is essential in interpreting spectroscopic data and understanding the electronic transitions that occur in excited states of atoms and molecules.
Quantum Mechanical Modeling: The differences between Px, Py, and Pz orbitals are fundamental to quantum mechanical models used in computational chemistry. These models rely on solving mathematical equations representing these orbitals to predict molecular properties, energies, and reaction mechanisms. Accurate descriptions of these orbitals are crucial for reliable computational simulations and calculations.
Understanding the differences between Px, Py, and Pz orbitals is essential for comprehending molecular geometry, bonding, electron configuration, spectroscopy, and computational modeling.
Science provides the basis to explain the behavior and properties of molecules and atoms, which leads to improvements in areas such as chemistry and materials research, and the discovery of drugs.
What are Px Orbitals?
The term “Px orbital” refers to one of the three p orbitals that belong to the second energy level (n = 2) of an atom. Px is the designation for the p orbital that is oriented along the x-axis.
Here are the key characteristics of the Px orbital:
Orientation: The Px orbital is aligned along its x-axis within a Cartesian coordinate system. P1 orbitals comprise one of three p orbitals; other examples being Py (on the y-axis) and Pz (along the z-axis).
Shape: The Px orbital has a dumbbell shape, with a nodal plane perpendicular to the x-axis. It consists of two lobes on either side of the nucleus along the x-axis. These lobes have opposite phases, indicating the opposite signs of the wave function.
Probability Distribution: The probability of finding an electron in Px orbitals increases along the x-axis; electron density gradually declines away from this region in both directions (y and z).
Quantum Numbers: The Px orbital has the same principal quantum number (n = 2) as the other two p orbitals (Py and Pz). The azimuthal quantum number (l) for all p orbitals is 1, indicating that they are p-type orbitals.
Perpendicularity: The Px orbital is perpendicular to both the Py and Pz orbitals. Together, these three p orbitals form a set of mutually perpendicular orbitals, contributing to a comprehensive three-dimensional description of the electron density distribution around the nucleus.
The Px orbital, along with the Py and Pz orbitals, plays a significant role in molecular bonding, determining the shape and geometry of molecules, and influencing the properties and behavior of electrons in chemical reactions. Understanding the Px orbital’s characteristics is essential for comprehending atomic structure, chemical bonding, and the properties of elements and compounds.
What is Py Orbitals?
The term “Py orbital” refers to one of the three p orbitals that belong to the second energy level (n = 2) of an atom. Py is the designation for the p orbital that is oriented along the y-axis.
Here are the key characteristics of the Py orbital:
Orientation: It is the Py orbital aligned to the y-axis of the Cartesian Coordinate System. This is the third of orbitals called p together with Px (oriented towards an x-axis) and Pz (oriented in the Z-axis).
Shape: The Py orbital has a dumbbell shape, with a nodal plane perpendicular to the y-axis. It is composed of two lobes: one above it, and the other below on the y-axis. These lobes have opposite phases, indicating the opposite signs of the wave function.
Probability distribution: The likelihood that an electron will be found within the Py orbital is greatest on the y-axis. The density of electrons decreases when you get further out from the direction of y, both in Z’s direction. as well as Z.
Quantum Numbers: The Py orbital has the same principal quantum number (n = 2) as the other two p orbitals (Px and Pz). The azimuthal quantum number (l) for all p orbitals is 1, indicating that they are p-type orbitals.
Perpendicularity: The Py orbital is perpendicular to both the Px and Pz orbitals. Together, these three p orbitals form a set of mutually perpendicular orbitals, contributing to a comprehensive three-dimensional description of the electron density distribution around the nucleus.
The Py orbital, along with the Px and Pz orbitals, is involved in molecular bonding, determining the shape and geometry of molecules, and influencing the properties and behavior of electrons in chemical reactions.
Understanding the characteristics of the Py orbital is essential for comprehending atomic structure, chemical bonding, and the properties of elements and compounds.
What is Pz Orbitals?
The Pz orbital is one of the three p orbitals that exist in an atom. The p orbitals (Px, Py, and Pz) belong to the second energy level (n = 2) and have specific orientations and shapes.
The Pz orbital is oriented along the z-axis, which is perpendicular to the x and y axes. It has a dumbbell shape with a node at the nucleus and two lobes above and below the nodal plane along the z-axis. The lobes are of equal size and have opposite phases, indicating opposite signs of the wave function.
The Pz orbital, along with the Px and Py orbitals, plays a crucial role in describing the electron distribution in three dimensions around an atom. These orbitals are involved in molecular bonding, determining the shape of molecules, and predicting bond angles.
In terms of energy, the Pz orbital has the same energy level as the Px and Py orbitals. Together, these three p orbitals complete the set of orbitals for the second energy level, providing a more comprehensive understanding of electron behavior in atoms and molecules.
The Pz orbital, along with the Px and Py orbitals, is essential in understanding molecular geometry, chemical bonding, and the behavior of electrons in chemical reactions.
Definition of Px, Py, and Pz Orbitals
Px, Py, and Pz orbitals are a set of three p orbitals that belong to the second energy level (n = 2) of an atom. Atomic orbitals, or regions around a nucleus where electrons tend to gather, are one type of atomic orbitals.
The definition of Px, Py, and Pz orbitals can be summarized as follows:
Px Orbital: Of all three P orbitals, Px is one that follows along its respective x-axis in a three-dimensional Cartesian coordinate system. It has a dumbbell shape with two nodes perpendicular to the x-axis and two lobes perpendicular to it; on either side of its nucleus are two prongs, giving this model its familiar silhouette.
Py Orbital: The Py orbital is another p orbital and is oriented along the y-axis. Like the Px orbital, it has a dumbbell shape, with a nodal plane perpendicular to the y-axis and two lobes above and below the nucleus along the y-axis.
Pz Orbital: The Pz orbital is the third p orbital and is oriented along the z-axis. It has a distinctive dumbbell shape with two nodes perpendicular to the z-axis and two lobes above and below its nucleus along its longitudinal axis.
The Px, Py, and Pz orbitals are part of the p subshell, which has an azimuthal quantum number (l) of 1. The principal quantum number (n) for these orbitals is 2, indicating that they belong to the second energy level. Each orbital can hold two electrons and their orientation contributes to a three-dimensional description of electron density distribution around an atom.
Understanding Px, Py, and Pz orbitals is fundamental in predicting molecular shapes, explaining chemical bonding, and studying electron configurations in atoms and molecules. These orbitals provide critical insights into the behavior and properties of matter at the atomic and molecular levels.
What is the Difference Between Px Py and Pz Orbitals?
The Px, Py, and Pz orbitals are three distinct p orbitals that have different orientations and shapes. Here are the key differences between them:
Orientation:
Px Orbital: The Px orbital is oriented along the x-axis.
Py Orbital: The Py orbital is oriented along the y-axis.
Pz Orbital: The Pz orbital is oriented along the z-axis.
Shape:
Px Orbital: The Px orbital has a dumbbell shape with a nodal plane perpendicular to the x-axis. It has two lobes, one of which is on each side of the nucleus on the x-axis.
Py Orbital: The Py orbital also has a dumbbell shape but with a nodal plane perpendicular to the y-axis. It is composed of two lobes, one higher than the nucleus and one below in the direction of y.
Pz Orbital: The Pz orbital has a dumbbell shape but with a nodal plane perpendicular to the z-axis. It consists of two lobes, one above and one below the nucleus along the z-axis.
Directionality:
Px Orbital: The electron density is concentrated along the x-axis and spread out in the y and z directions.
Py Orbital: The electron density is concentrated along the y-axis and spread out in the x and z directions.
Pz Orbital: The electron density is concentrated along the z-axis and spread out in the x and y directions.
Quantum Numbers:
Px, Py, and Pz orbitals all have the same principal quantum number (n = 2) since they belong to the second energy level.
The azimuthal quantum number (l) is the same for all three orbitals (l = 1), indicating that they are p orbitals.
The magnetic quantum number (m) differentiates between the three orbitals. For Px, m = 0, for Py, m = ±1, and for Pz, m = 0.
Spatial Relationship:
Px, Py, and Pz orbitals are mutually perpendicular to each other. The axes aligned are the x, y, and Z axes making an x, y, and z coordinate system.
These differences are essential in understanding molecular geometry, chemical bonding, and the behavior of electrons in atomic and molecular systems. The Px, Py, and Pz orbitals contribute to the overall electron density distribution around the atom, influencing the shape and properties of molecules in various chemical reactions.
What are the similarities between Px Py and Pz Orbitals?
Despite their distinct orientations and shapes, the Px, Py, and Pz orbitals also share several similarities:
Energy Level: Px, Py, and Pz orbitals all belong to the same energy level in an atom. They are part of the second energy level (n = 2).
Angular Momentum Quantum Number: The Px, Py, and Pz orbitals share the same azimuthal quantum number (l = 1). This means that they are all p orbitals, which have dumbbell-like shapes.
Number of Nodes: Each of the three orbitals has one node at the nucleus. A node is a region of zero electron density, and it separates the two lobes of the dumbbell-shaped orbitals.
Lobes and Phases: The Px, Py, and Pz orbitals all have two lobes. In each orbital, one lobe is positive in phase (+) while the other is negative in phase (-). The nodes separate these lobes with opposite phases.
Perpendicularity: The Px, Py, and Pz orbitals are mutually perpendicular to each other. The Px orbital is perpendicular to both the Py and Pz orbitals, and similarly, the Py orbital is perpendicular to both the Px and Pz orbitals. This perpendicularity forms a three-dimensional coordinate system.
Involvement in Bonding: All three orbitals participate in molecular bonding and hybridization. In chemical reactions, they can overlap with orbitals from other atoms, leading to the formation of covalent bonds.
Probability Distribution: The probability distribution of finding an electron in the Px, Py, and Pz orbitals is highest along the axes along which they are oriented (x, y, and z, respectively).
Quantum Mechanics: Px, Py, and Pz orbitals are described by quantum mechanics and quantum numbers. The principal quantum number (n) for all three orbitals is the same, and they share the same magnetic quantum number (m = 0 for Px and Pz, m = ±1 for Py).
These similarities allow Px, Py, and Pz orbitals to collectively describe the electron density distribution in three-dimensional space around the nucleus of an atom. Understanding these common aspects is vital for comprehending molecular bonding, molecular geometry, and the behavior of electrons in chemical reactions.
Px vs Py vs Pz Orbitals in Tabular Form
Below is a tabular comparison of the key characteristics of Px, Py, and Pz orbitals:
| Characteristic | Px Orbital | Py Orbital | Pz Orbital |
|---|---|---|---|
| Orientation | Along the x-axis | Along the y-axis | Along the z-axis |
| Shape | Dumbbell-shaped | Dumbbell-shaped | Dumbbell-shaped |
| Nodal Plane | Perpendicular to x-axis | Perpendicular to y-axis | Perpendicular to z-axis |
| Lobes | Two lobes along X-axis | Two lobes along the y-axis | Two lobes along the z-axis |
| Probability | Highest along x-axis | Highest along y-axis | Highest along z-axis |
| Distribution | Decreases away from the x-axis | Decreases away from the y-axis | Decreases away from the z-axis |
| Quantum Numbers | n = 2, l = 1, m = 0 | n = 2, l = 1, m = ±1 | n = 2, l = 1, m = 0 |
| Perpendicularity | Perpendicular to Py and Pz | Perpendicular to Px and Pz | Perpendicular to Px and Py |
| Involvement in Bonding | Participates in molecular bonding and hybridization. | Participates in molecular bonding and hybridization. | Participates in molecular bonding and hybridization. |
Note: In the “Quantum Numbers” row, “n” represents the principal quantum number while “l” and “m” stand for azimuthal and magnetic quantum numbers, respectively. For Px and Pz, the value of “m” is 0, while for Py, “m” can take values of ±1.
The Px, Py, and Pz orbitals are collectively known as the “p orbitals” and form a set of mutually perpendicular orbitals, contributing to the three-dimensional electron density distribution around the nucleus.
Examples and Applications
Examples and applications of Px, Py, and Pz orbitals in chemistry and other scientific fields include:
Molecular Geometry: Px Py orbitals and Pz are crucial in determining the three-dimensional form and shape of molecules. For example, in molecules with sp² hybridization (e.g., ethene), the Px and Py orbitals form sigma bonds, while the Pz orbital forms a pi bond.
Molecular Bonding: In molecules like ammonia (NH₃), the Px and Py orbitals of the nitrogen atom overlap with the hydrogen 1s orbitals, leading to the formation of sigma bonds in the ammonia molecule.
Electron Configuration: The Px, Py, and Pz orbitals contribute to the electron configuration of atoms and ions. For example, a carbon atom in its ground state has two electrons in the 2s orbital and two electrons in the Px and Py orbitals (2p²).
Molecular Orbital Theory: In molecular orbital theory, Px, Py, and Pz orbitals combine to form molecular orbitals. The linear combination of these orbitals helps in understanding the bonding and stability of molecules.
Excited States: In spectroscopy, the transition of electrons between Px, Py, and Pz orbitals can result in the absorption or emission of specific wavelengths of light. This phenomenon helps identify elements and study the electronic structure of molecules.
Chemical Reactions: Px, Py, and Pz orbitals determine the overlap and interaction of atomic orbitals during chemical reactions. Understanding these interactions helps explain reaction mechanisms and predict reaction products.
Magnetic Resonance Imaging (MRI): MRI relies on the behavior of protons in the Px, Py, and Pz orbitals of hydrogen nuclei. By manipulating the alignment of these protons in a magnetic field and measuring their relaxation, MRI provides detailed images of tissues in the human body.
Molecular Modeling: Px, Py, and Pz orbitals are essential in computational chemistry and molecular modeling. These orbitals are used in quantum mechanical calculations to predict molecular properties and study molecular dynamics.
Nanotechnology: The behavior of electrons in Px, Py, and Pz orbitals is fundamental in understanding and designing nanoscale materials and devices.
Crystallography: The arrangement of atoms in crystals can be analyzed using diffraction methods, which rely on the electron density distributions determined by the Px, Py, and Pz orbitals.
The Px, Py, and Pz orbitals have broad applications across various scientific disciplines, including chemistry, spectroscopy, imaging, material science, and quantum mechanics. They offer important insights into molecular structure, properties, and reactivity. They are crucial tools for studying and manipulating matter at the molecular and atomic levels.
Conclusion
The Px, Py, and Pz orbitals are a set of three p orbitals that play a fundamental role in describing the electron distribution around an atom and in understanding the properties and behavior of molecules.
These orbitals belong to the second energy level (n = 2) and have distinct orientations and shapes. Px is located along the x-axis. Py in the direction of the y-axis Pz along the y-axis, and Pz along the Z-axis.
The differences between Px, Py, and Pz orbitals lie in their orientations, shapes, and spatial distributions. However, they also share similarities, such as belonging to the same energy level, having one node at the nucleus, and participating in molecular bonding and hybridization.