Organic chemistry offers many fascinating reactions that determine how molecules behave, making the subject truly fascinating and accessible for students of any background. Among these reactions, Nucleophilic and Electrophilic additions hold significant importance. They are fundamental processes that underpin various chemical transformations, playing a vital role in the synthesis of complex molecules.
A brief overview of addition reactions in organic chemistry
In organic chemistry, addition reactions are a fundamental class of chemical transformations where two or more reactants combine to form a single, larger molecule. The reaction involves adding atoms or groups of atoms to a substrate, often leading to new functional groups being formed.
Addition reactions are widely prevalent in organic chemistry and play a crucial role in the synthesis of complex molecules and the functionalization of organic compounds.
Key characteristics of addition reactions include:
1. Substrate: The molecule to which the addition occurs is referred to as the substrate. It typically contains a multiple bond, such as a double bond (alkene) or a triple bond (alkyne), which provides the necessary electron-rich region for the addition of atoms or groups.
2. Reactants: Addition reactions involve two or more reactants. Individual or groups of atoms called reagents, are added to a substrate for analysis.
Electrophiles and Nucleophiles: Addition reactions can be classified based on the nature of the reacting species. Electrophiles are electron-deficient species that seek electrons to complete their valence shells. Nucleophiles, on the other hand, are electron-rich species that donate their lone pairs of electrons to form new bonds.
3. Mechanism: An addition reaction’s mechanism depends on the reaction type. Nucleophilic addition involves attacking electron-deficient centers with nucleophiles while electrophilic expansion involves adding electrophiles onto electron-rich centers.
4. Regioselectivity and Stereoselectivity: Addition reactions may exhibit regioselectivity, which refers to the preference for adding atoms or groups to specific positions in the substrate. Stereoselectivity, on the other hand, concerns the spatial arrangement of the newly formed stereocenters in the product.
5. Catalysts: Some additional reactions may require the presence of catalysts to facilitate the reaction or to improve its efficiency.
Addition reactions are integral to various organic transformations, such as the synthesis of alcohols, carbonyl compounds, halides, and many other functional groups. Understanding the mechanisms and selectivities of addition reactions is essential for organic chemists to design and control the synthesis of target molecules with specific structures and properties.
Importance of understanding nucleophilic and electrophilic additions
Understanding nucleophilic and electrophilic additions is of significant importance in organic chemistry due to the following reasons:
Predicting Reactivity: Chemistry allows chemists to better predict the reactions of various molecules through an understanding of nucleophilic and electrophilic additions. By identifying nucleophilic and electrophilic centers in substrates, they can anticipate the sites of potential reactions and design appropriate reaction conditions.
1. Reaction Design and Optimization: Organic chemists can use this understanding to design and optimize synthetic routes for the targeted formation of specific functional groups. They can select suitable nucleophiles and electrophiles to achieve the desired transformations efficiently.
2. Organic Synthesis: Nucleophilic and electrophilic additions are key reactions in organic synthesis. These reactions result in complex molecules like pharmaceuticals, agrochemicals, and natural products which play an essential part in medicine, agriculture, and other areas.
Regioselectivity and Stereoselectivity: By understanding the factors that control regioselectivity (site-selectivity) and stereoselectivity (stereoselective outcome) in nucleophilic and electrophilic additions, chemists can manipulate reactions to obtain specific products with desired region- and stereochemistry.
3. Mechanistic Insights: Studying nucleophilic and electrophilic additions provides valuable mechanistic insights into the reactivity of various functional groups. This knowledge aids in understanding reaction pathways and helps chemists propose and validate reaction mechanisms.
4. Reaction Optimization: Understanding nucleophilic and electrophilic additions allows chemists to optimize reaction conditions, including temperature, solvent, and catalysts. This optimization can enhance reaction yields and reduce unwanted side reactions.
5. Reaction Control: Controlling nucleophilic and electrophilic additions is crucial in achieving high selectivity and minimizing waste. Chemists can apply their knowledge to steer reactions toward the desired products while avoiding undesired byproducts.
6. Medicinal Chemistry: In drug design, knowledge of nucleophilic and electrophilic additions is vital for the functionalization of pharmaceutical molecules. By selectively modifying specific functional groups, chemists can enhance drug potency, stability, and pharmacological properties.
7. Material Science: Understanding nucleophilic and electrophilic additions is relevant in material science for the functionalization and modification of polymers, resins, and other materials to obtain desired properties.
Understanding nucleophilic and electrophilic additions empowers chemists to control and manipulate chemical reactions, design efficient synthetic routes, and construct complex molecules with high selectivity and specificity. This knowledge is indispensable for various fields, including drug development, materials science, and the synthesis of essential compounds for societal needs.
Nucleophilic Addition
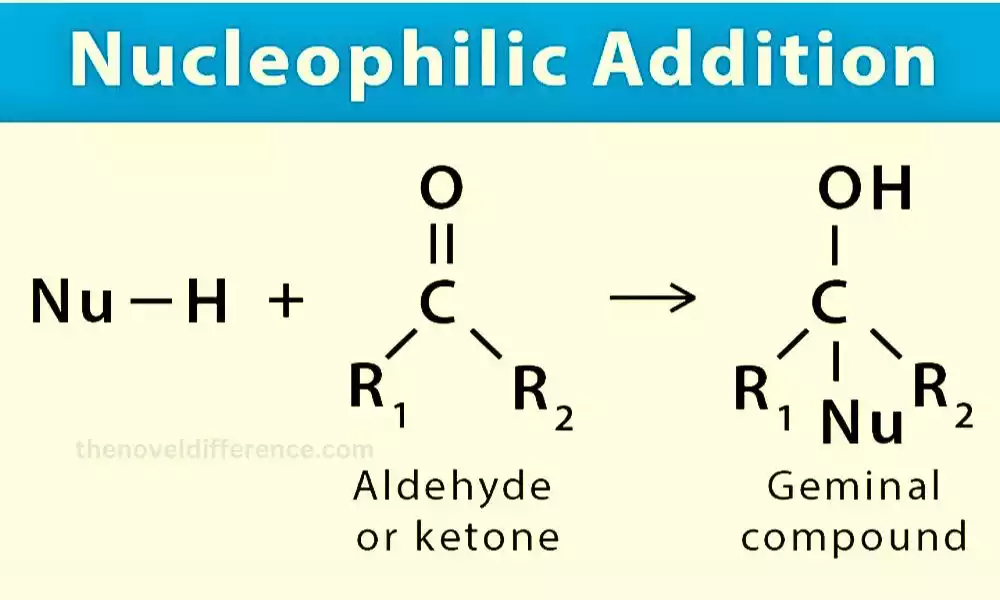
Nucleophilic addition is an organic chemical reaction where nucleophiles react with substrates to form new covalent bonds by attaching themselves as nucleophiles to them and adding nucleophilic agents directly. Nucleophiles are electron-rich species, typically possessing lone pairs of electrons or π-electrons, which they can donate to an electron-deficient center in the substrate.
The general mechanism of nucleophilic addition involves the following steps:
1. Nucleophile Attack: The nucleophile approaches the electron-deficient center in the substrate, which is often a π-bond (such as a double or triple bond) or a partially positive atom.
1. Formation of a New Bond: When nucleophiles donate an electron pair, resulting in covalent bonds are formed between themselves and the substrate.
Charge Rearrangement: As the nucleophile forms a bond, the electron-deficient center (often a carbon atom) becomes negatively charged, and the nucleophile’s atom gains a positive charge or partial positive charge.
Product Formation: The final product of the nucleophilic addition reaction is a new compound with the nucleophile incorporated into the substrate.
Examples of nucleophilic addition reactions include:
Addition of Water to an Alkene:
CH2=CH2 + H2O → CH3CH2OH
Addition of Ammonia to a Carbonyl Compound (Imine Formation):
RCHO + NH3 → RCH=NH + H2O
Addition of Hydrogen Cyanide to a Carbonyl Compound (Cyanohydrin Formation):
RCHO + HCN → RCH(OH)CN
Nucleophilic addition reactions are fundamental in organic chemistry and are widely used in organic synthesis to build complex molecules, functionalize organic compounds, and introduce specific functional groups into the target molecules.
Nucleophilic addition reactions may differ according to various factors such as substrate nature and strength of nucleophile as well as reaction conditions. Understanding nucleophilic addition is essential for designing efficient and selective synthetic routes in organic chemistry.
Definition of nucleophilic addition
Nucleophilic addition is a chemical process in organic chemistry in which nucleophiles react with substrates to add them and form new covalent bonds, leading to nucleophilic addition. Nucleophiles are electron-rich species that contain lone pairs of electrons or π-electrons, which they can donate to an electron-deficient center in the substrate.
The nucleophile attacks an electron-deficient region in the substrate, which is often a π-bond (e.g., a double or triple bond) or a partially positive atom. Substantial electron donation from the nucleophile to the substrate leads to the formation of covalent bonds between these substances, providing energy transfer between nucleophiles and substrates.
The general mechanism of nucleophilic addition involves the following steps:
1. Nucleophile Attack: The nucleophile approaches the electron-deficient center in the substrate.
2. Formation of a New Bond: The nucleophile donates its electron pair to the electron-deficient center, resulting in the formation of a new covalent bond.
3. Charge Rearrangement: As the nucleophile forms a bond, the electron-deficient center (often a carbon atom) becomes negatively charged, and the nucleophile’s atom gains a positive charge or partial positive charge.
4. Product Formation: The final product of the nucleophilic addition reaction is a new compound with the nucleophile incorporated into the substrate.
Nucleophilic addition reactions are fundamental in organic chemistry and are widely used in organic synthesis to construct complex molecules, functionalize organic compounds, and introduce specific functional groups into the target molecules.
Nucleophilic addition reactions are determined by several variables, including substrate material, the strength of nucleophiles, and reaction conditions. These considerations can significantly impact their selectivity and regiochemistry. Understanding nucleophilic addition is essential for designing efficient and selective synthetic routes in organic chemistry.
Mechanism of nucleophilic additions
Nucleophilic addition involves attacking electron-deficient sites within a substrate with nucleophile molecules to form covalent bonds between nucleophile and substrate molecules.
Nucleophilic additions are common in organic chemistry and play a crucial role in various reactions, including those with carbonyl compounds, alkenes, and alkynes. The mechanism can vary depending on the specific reaction and the nature of the substrate and nucleophile involved.
Here, we’ll outline the general mechanism of nucleophilic addition to carbonyl compounds and alkenes:
Nucleophilic Addition to Carbonyl Compounds:
Carbonyl compounds like aldehydes and ketones contain carbon-oxygen bonds with electron-deficient centers susceptible to being attacked by nucleophilic reactions.
1. General Steps:
a. Nucleophilic Attack: The nucleophile (often a negatively charged species or a species with a lone pair of electrons) approaches the electrophilic carbon of the carbonyl group, which is partially positively charged due to the electron-withdrawing effect of the oxygen.
b. Formation of Tetrahedral Intermediate: The nucleophile donates its lone pair of electrons to the electrophilic carbon, forming a tetrahedral intermediate. The oxygen atom of the carbonyl group gains a negative charge during this step.
c. Deprotonation or Proton Transfer: Deprotonation or proton transfer may sometimes occur to reduce negatively charged oxygen atoms to balance out their charges and ensure their stability. This step leads to the formation of the final product.
Example: Nucleophilic addition of a hydride ion (H-) to a carbonyl compound (aldehyde or ketone) forming an alcohol:
R-C=O + H- → R-C-OH
2. Nucleophilic Addition to Alkenes:
Alkenes contain a C=C double bond, which is the electron-rich region available for nucleophilic addition.
3. General Steps:
a. Nucleophilic Attack: The nucleophile approaches the electrophilic carbon of the double bond, which has a higher electron density due to the π-bond.
b. Formation of a Sigma Complex: The nucleophile donates its lone pair of electrons to one of the carbon atoms involved in the double bond. This leads to the formation of a sigma complex, where the two carbons are connected by a single bond.
c. Proton Transfer and Rearrangement (optional): In some cases, proton transfer or rearrangement may occur to stabilize the intermediate and form the final product.
Example: Nucleophilic addition of a hydroxide ion (OH-) to an alkene forming an alcohol:
CH2=CH2 + OH- → CH3-CH2OH
Nucleophilic addition can vary depending on the nature and concentration of its nucleophile component, the substrate material used, and reaction conditions. Understanding the mechanisms of nucleophilic additions is crucial for designing efficient and selective synthetic routes in organic chemistry.
Electrophilic Addition
Electrophilic addition is an organic chemical reaction where an electrophile reacts with a substrate, leading to its addition and the creation of new covalent bonds. Electrophiles are electron-deficient species that are attracted to regions of high electron density in the substrate.
The general mechanism of electrophilic addition involves the following steps:
Electrophile Attack: The electrophile approaches the region of high electron density in the substrate, which is often a π-bond (e.g., a double or triple bond) or a lone pair of electrons on an atom.
Formation of a New Bond: An electrophile reacts by accepting electrons from regions with high electron densities in its substrate. It bonds them directly with that material, creating new covalent bonds between itself and that substrate.
Charge Rearrangement: As the electrophile forms a bond, the region in the substrate from which the electrons were donated becomes positively charged or partially positive, and the electrophile may gain a negative charge or partial negative charge.
Product Formation: The final product of the electrophilic addition reaction is a new compound with the electrophile incorporated into the substrate.
Examples of electrophilic addition reactions include:
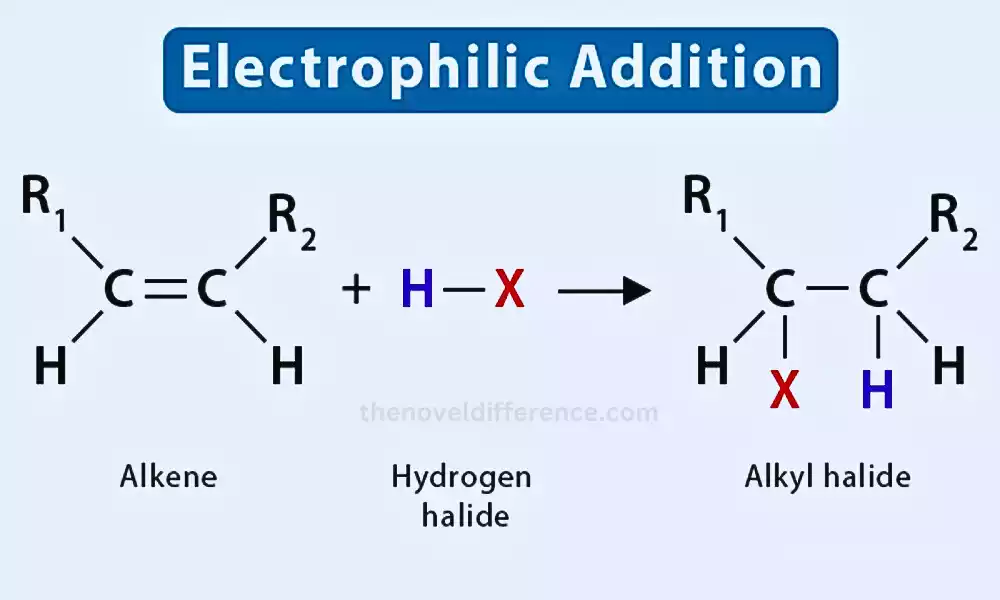
1. Addition of Hydrogen Halides to Alkenes:
CH2=CH2 + HX → CH3-CH2X (where X = halogen)
2. Addition of Electrophilic Reagents to Alkynes:
HC≡CH + X2 → HC≡CX-X (where X = halogen)
3. Addition of Electrophiles to Benzene (Aromatic Electrophilic Substitution):
C6H6 + E → C6H5-E (where E = electrophile)
Electrophilic addition reactions are common in organic chemistry and are particularly significant in reactions involving alkenes and alkynes. These reactions are vital in the functionalization of organic molecules, allowing the introduction of various functional groups and the construction of more complex structures.
The selectivity and regiochemistry of electrophilic addition reactions can be influenced by factors such as the nature of the substrate, the strength of the electrophile, and the reaction conditions. Understanding electrophilic addition reactions is essential for designing efficient and selective synthetic routes in organic chemistry.
Definition of electrophilic addition
Electrophilic addition is a type of chemical reaction in organic chemistry in which an electrophile reacts with a substrate to form covalent bonds with it and add an electrophile, leading to further chemical modification on either end. Electrophiles are electron-deficient species that are attracted to regions of high electron density in the substrate.
The electrophile attacks an electron-rich region in the substrate, which is often a π-bond (e.g., a double or triple bond) or a lone pair of electrons on an atom. Donating electrons from a substrate to an electrophile leads to the formation of new covalent bonds between those elements and the substrate.
The general mechanism of electrophilic addition involves the following steps:
Electrophile Attack: The electrophile approaches the electron-rich region in the substrate, which has a higher electron density due to the presence of π-electrons or a lone pair of electrons.
Formation of a New Bond: When an electrophile accepts electrons from electron-rich regions within its substrate, this causes it to form covalent bonds with this region, thus creating new covalent bonds between the electrophile and substrate.
1. Charge Rearrangement: As the electrophile forms a bond, the region in the substrate from which the electrons were donated becomes positively charged or partially positive, and the electrophile may gain a negative charge or partial negative charge.
2. Product Formation: The final product of the electrophilic addition reaction is a new compound with the electrophile incorporated into the substrate.
3. Examples of electrophilic addition reactions include:
Addition of Hydrogen Halides to Alkenes:
CH2=CH2 + HX → CH3-CH2X (where X = halogen)
Addition of Electrophilic Reagents to Alkynes:
HC≡CH + X2 → HC≡CX-X (where X = halogen)
Addition of Electrophiles to Benzene (Aromatic Electrophilic Substitution):
C6H6 + E → C6H5-E (where E = electrophile)
Electrophilic addition reactions are essential in organic chemistry and play a crucial role in the functionalization of organic molecules, allowing the introduction of various functional groups and the construction of more complex structures.
The selectivity and regiochemistry of electrophilic addition reactions can be influenced by factors such as the nature of the substrate, the strength of the electrophile, and the reaction conditions. Understanding electrophilic addition reactions is vital for designing efficient and selective synthetic routes in organic chemistry.
Mechanism of electrophilic additions
Electrophilic addition involves attacking an electron-rich center of a substrate with an electrophile, creating a covalent bond between themselves and said substrate. Electrophilic additions are common in organic chemistry and are particularly significant in reactions involving alkenes and alkynes.
The mechanism can vary depending on the specific reaction and the nature of the substrate and electrophile involved.
Here, we’ll discuss the general means of electrophilic additions to alkenes and alkynes:
Electrophilic Addition to Alkenes:
Alkenes contain a C=C double bond, which is the electron-rich region available for electrophilic attack.
General Steps:
a. Electrophile Approach: The electrophile approaches the π-bond of the alkene, attracted by the high electron density in the region between the two carbon atoms.
b. Formation of a Sigma Complex: The electrophile accepts a pair of electrons from one of the carbon atoms involved in the double bond, forming a Sigma complex. In this complex, the two carbons are connected by a single bond with the electrophile temporarily attached to one of the carbon atoms.
c. Proton Transfer and Rearrangement (optional): In some cases, proton transfer or rearrangement may occur to stabilize the intermediate and form the final product.
Example: Electrophilic addition of a hydrogen halide (HX) to an alkene forming an alkyl halide:
CH2=CH2 + HX → CH3-CH2X (where X = halogen)
Electrophilic Addition to Alkynes:
Alkynes contain a C≡C triple bond, which is the electron-rich region available for electrophilic attack.
General Steps:
a. Electrophile Approach: The electrophile approaches the triple bond of the alkyne, attracted by the high electron density in the region between the two carbon atoms.
b. Formation of a Sigma Complex: The electrophile accepts a pair of electrons from one of the carbon atoms involved in the triple bond, forming a Sigma complex. The two carbons are connected by a single bond with the electrophile temporarily attached to one of the carbon atoms.
c. Proton Transfer and Rearrangement (optional): In some cases, proton transfer or rearrangement may occur to stabilize the intermediate and form the final product.
Example: Electrophilic addition of a halogen (X2) to an alkyne forming a geminal dihalide:
HC≡CH + X2 → HC≡CX-X (where X = halogen)
Electrophilic addition can take place through various mechanisms depending on the election trophic conditions substrate material, and reaction conditions. Understanding the mechanisms of electrophilic additions is crucial for designing efficient and selective synthetic routes in organic chemistry.
Comparison Between Nucleophilic and Electrophilic Additions
Nucleophilic and electrophilic additions are two important types of addition reactions in organic chemistry. While they both involve the addition of a species to a substrate, they differ in terms of the reacting species, the type of reaction, regioselectivity, and reaction conditions.
Here’s a comparison between nucleophilic and electrophilic additions:
1. Reacting Species:
Nucleophilic Addition: In nucleophilic additions, the reacting species is a nucleophile, which is an electron-rich species containing lone pairs of electrons or π-electrons. Nucleophiles donate electrons to an electron-deficient center in the substrate.
Electrophilic Addition: In electrophilic additions, the reacting species is an electrophile, which is an electron-deficient species that seeks electrons to complete its valence shell. Electrophiles accept electrons from an electron-rich center in the substrate.
2. Type of Reaction:
Nucleophilic Addition: Nucleophilic Additions are the process of applying nucleophiles on substrates to form covalent bonding that will ultimately join them.
Electrophilic Addition: Electrophilic Addition is the process of attaching electrophiles onto substrates in order to produce new covalent bonds.
3. Regioselectivity and Stereoselectivity:
Nucleophilic Addition: Nucleophilic additions can exhibit regioselectivity, which means they may add to specific positions in the substrate based on the electron density. Stereoselectivity may also be observed, leading to the formation of particular stereoisomers in the product.
Electrophilic Addition: Electrophilic additions can also exhibit regioselectivity and stereoselectivity, where the electrophile may preferentially add to specific positions in the substrate and form specific stereoisomers in the product.
4. Reaction Conditions and Catalysts:
Nucleophilic Addition: Nucleophilic additions typically require the presence of a nucleophile and may occur under basic or nucleophilic reaction conditions.
Electrophilic Addition: Electrophilic additions require the presence of an electrophile and may occur under acidic or electrophilic reaction conditions.
5. Substrate Preference:
Nucleophilic Addition: Nucleophilic additions frequently take place with substrates containing either an alkene (alkenes and alkynes) or carbonyl compounds with an electronegative bond or functional group (carbonyl compounds).
Electrophilic Addition: Electrophilic additions frequently take place with substrates containing either p-bonds (alkenes and alkynes) or aromatic compounds such as benzene.
6. Importance in Organic Synthesis:
Nucleophilic Addition: Nucleophilic additions play an integral part in synthesizing alcohols, amines, and other functional groups found within organic molecules. They are widely used in organic synthesis for the construction of complex molecules.
Electrophilic Addition: Electrophilic additions are crucial for the functionalization of organic molecules, allowing the introduction of various functional groups and the construction of more complex structures.
Both nucleophilic and electrophilic additions are vital reactions in organic chemistry, and understanding their mechanisms and selectivities is essential for designing efficient and selective synthetic routes to create a wide range of organic compounds with specific functionalities.
Applications in Industries and Everyday Life
Nucleophilic and electrophilic additions have numerous applications in industries and everyday life. These addition reactions are essential tools in organic synthesis and play a vital role in producing various products that impact our daily lives. Here are some of the applications of nucleophilic and electrophilic additions:
1. Pharmaceutical Industry:
Nucleophilic additions are used to introduce functional groups in drug molecules, such as the addition of amines to carbonyl compounds to form amides or the addition of nucleophiles to carbon-carbon double bonds to create new chiral centers. These reactions are crucial for the synthesis of many pharmaceutical compounds.
2. Agrochemical Industry:
Synthesis of pesticides and herbicides is done using nucleophilic and electrophilic additions. These reactions enable the functionalization of organic compounds to enhance their efficacy in controlling pests and diseases in agriculture.
3. Petrochemical Industry:
Nucleophilic additives are often employed in the production of petrochemical products like resins, plastics, and synthetic rubber. These reactions allow the modification of petroleum-based feedstocks to produce a wide range of materials used in everyday products.
4. Polymer Chemistry:
Nucleophilic and electrophilic additions play a crucial role in polymerization reactions, where monomers undergo addition reactions to form long polymer chains. These reactions are fundamental in the production of various synthetic polymers used in plastics, fibers, adhesives, and coatings.
5. Flavor and Fragrance Industry:
Nucleophilic and electrophilic additions are employed to synthesize flavor compounds, fragrances, and aroma chemicals. These reactions are essential for introducing specific functional groups and creating a diverse range of scents and flavors used in perfumes, cosmetics, and food products.
6. Organic Solvents:
Nucleophilic and electrophilic addition reactions are involved in the production of various organic solvents used in laboratories, industries, and everyday cleaning products.
7. Pharmaceutical Intermediates:
Nucleophilic and electrophilic additions are used to synthesize intermediate compounds that are further transformed into active pharmaceutical ingredients (APIs). These intermediate compounds serve as key building blocks in pharmaceutical synthesis.
8. Food Additives:
Food additives and preservatives can be made using nucleophilic or electrophilic additions. This improves the texture and taste of foods, as well as their shelf life.
9. Environmental Applications:
Nucleophilic and electrophilic additions are used in environmental remediation processes to degrade pollutants and detoxify hazardous chemicals.
The applications of nucleophilic and electrophilic additions are ubiquitous.
From the medicines we take to the materials we use, these addition reactions are integral to the production of a wide range of products that enhance our quality of life and drive technological advancements in various industries.
Challenges and Limitations of Nucleophilic and Electrophilic Addition
Nucleophilic and electrophilic additions are powerful and versatile reactions in organic chemistry, but they also come with certain challenges and limitations.
Here are some of the key challenges and limitations associated with these additional reactions:
Challenges of Nucleophilic Addition:
Reactivity of Substrates: Nucleophilic additions may not be feasible with all substrates. Some substrates may lack suitable electron-deficient centers for nucleophilic attack, limiting the scope of these reactions.
Stereoselectivity: Nucleophilic additions to chiral substrates can sometimes result in the formation of a mixture of stereoisomers. Achieving high stereoselectivity can be challenging, particularly in cases where multiple chiral centers are involved.
Nucleophile Reactivity: The reactivity of nucleophiles can vary significantly, and some nucleophiles may not be strong enough to undergo the desired addition reaction. This may necessitate the use of more reactive or specialized nucleophiles, which can add to the complexity of the reaction.
Side Reactions: Nucleophilic additions may sometimes lead to side reactions, such as elimination or competing substitution reactions, which can reduce the yield of the desired product.
1. Challenges of Electrophilic Addition:
Reactivity of Electrophiles: Electrophilic additions may not be suitable for all electrophiles, especially when the electrophile is too reactive or too unstable to undergo the desired addition reaction.
Regioselectivity: Achieving regioselectivity in electrophilic additions can be challenging, particularly when the substrate has multiple reactive sites. The electrophile may react at different positions in the substrate, leading to the formation of multiple products.
Rearrangement: In some cases, electrophilic additions may be accompanied by rearrangement reactions, leading to the formation of unexpected products.
Byproduct Formation: Electrophilic additions may result in the formation of byproducts, such as side products or waste compounds, which can reduce the overall efficiency of the reaction.
2. Both Nucleophilic and Electrophilic Additions:
Reaction Conditions: Nucleophilic and electrophilic additions may require specific reaction conditions, such as appropriate solvents, temperature, and catalysts. Finding the optimal reaction conditions can be time-consuming and challenging.
Selectivity: Achieving high selectivity in both nucleophilic and electrophilic additions, particularly when multiple reactive sites are present in the substrate, can be challenging. Controlling the reaction to obtain the desired product while minimizing the formation of undesired byproducts is crucial.
Functional Group Tolerance: Some functional groups in the substrate or nucleophile/electrophile may interfere with the reaction, leading to side reactions or low yields.
Despite these challenges and limitations, nucleophilic and electrophilic additions remain invaluable tools in organic synthesis and are continuously being studied and optimized to overcome their limitations and expand their applications. Chemists continue to develop new methodologies and strategies to improve the efficiency, selectivity, and scope of these important addition reactions.
Conclusion
Nucleophilic and electrophilic additions are fundamental addition reactions in organic chemistry that play a crucial role in the construction of complex molecules and the functionalization of organic compounds.
Nucleophilic additions involve the attack of a nucleophile on an electron-deficient center, resulting in the formation of a new covalent bond. Electrophilic additions involve attacking an electron-rich center with an electrophile to form a new covalent bond, leading to its formation and subsequent chemical reactions.
Nucleophilic and electrophilic additions find widespread application across industries such as pharmaceuticals, agrochemicals, petrochemicals, and materials science. These reactions are essential for synthesizing pharmaceuticals, pesticides, plastics, and numerous everyday products that enhance our lives and drive technological advancements.
