When it comes to the world of chemistry and chemical reactions, the concepts of buffer action and buffer capacity play a crucial role. Understanding the difference between buffer action and buffer capacity is essential for anyone studying or working in the field of chemistry. We will delve into the intricacies of buffer action and buffer capacity, explaining their definitions, and functions, and highlighting the key differences between the two concepts.
Definition of Buffer Action and Buffer Capacity
Buffer Action: Buffer action is like having a superhero squad that ensures pH stability in a solution. When an acid or base tries to mess with the pH, these buffers come to the rescue.
Here’s how it works Buffer action relies on a dynamic equilibrium between a weak acid and its partner, the conjugate base (or a weak base and its conjugate acid). These dynamic duos work together to neutralize any changes in pH caused by the intruder. They act as a shield, keeping the pH relatively stable.
Buffer Capacity: Now, let’s talk about buffer capacity. Imagine it as the buffer’s power to handle pH disturbances. The greater the buffer capacity, the better it can absorb and neutralize added acid or base.
Buffer capacity is determined by several factors. One important factor is the concentration of the weak acid and its conjugate base (or weak base and its conjugate acid). The more buffer components there are, the stronger the buffer’s ability to absorb acid or base without significant changes in pH. Other factors, such as the pH range, temperature, ionic strength, and interfering substances, can also influence buffer capacity.
Importance of Buffer Action and Buffer Capacity
Now that we have a solid understanding of buffer action and buffer capacity.
Let’s explore why they are so crucial in various contexts:
1. Applications in Science and Industry: Buffer systems are widely used in scientific research, medical laboratories, and industrial processes. They help maintain stable conditions for chemical reactions, enzymatic processes, and DNA/RNA experiments, to name just a few.
2. Biological Systems: Buffers play a pivotal role in maintaining the pH of our blood, cells, and organs. Without proper pH regulation, our bodies’ essential biochemical reactions would be compromised.
3. Stability in Chemical Reactions: Buffer systems are essential for controlling pH in chemical reactions. By keeping the pH stable, buffer action and buffer capacity ensure that reactions proceed smoothly, yielding consistent and reliable results.
What is Buffer Action?
Buffer action, also known as buffering, refers to the ability of a buffer solution to resist changes in pH. pH is a measure of the acidity or alkalinity of a solution and is determined by the concentration of hydrogen ions (H+) present. Buffer solutions are made up of a weak acid and its conjugate base (or a weak base and its conjugate acid), which work together to maintain a relatively stable pH.
When an acid or base is added to a buffer solution, the buffer action comes into play. The weak acid or base present in the buffer reacts with the added acid or base, neutralizing their effects. This reaction helps in preventing large fluctuations in pH and maintains the solution’s stability.
To better understand buffer action, let’s take a common example: the bicarbonate buffer system in our blood. Carbonic acid (H2CO3) and bicarbonate ions (HCO3-) form this buffer system. When excess acid is introduced into the blood, the bicarbonate ions combine with hydrogen ions, forming carbonic acid. Conversely, when an excess base is present, carbonic acid dissociates, releasing bicarbonate ions and neutralizing the excess base. This buffering system helps to maintain the blood’s pH within a narrow range, ensuring proper physiological function.
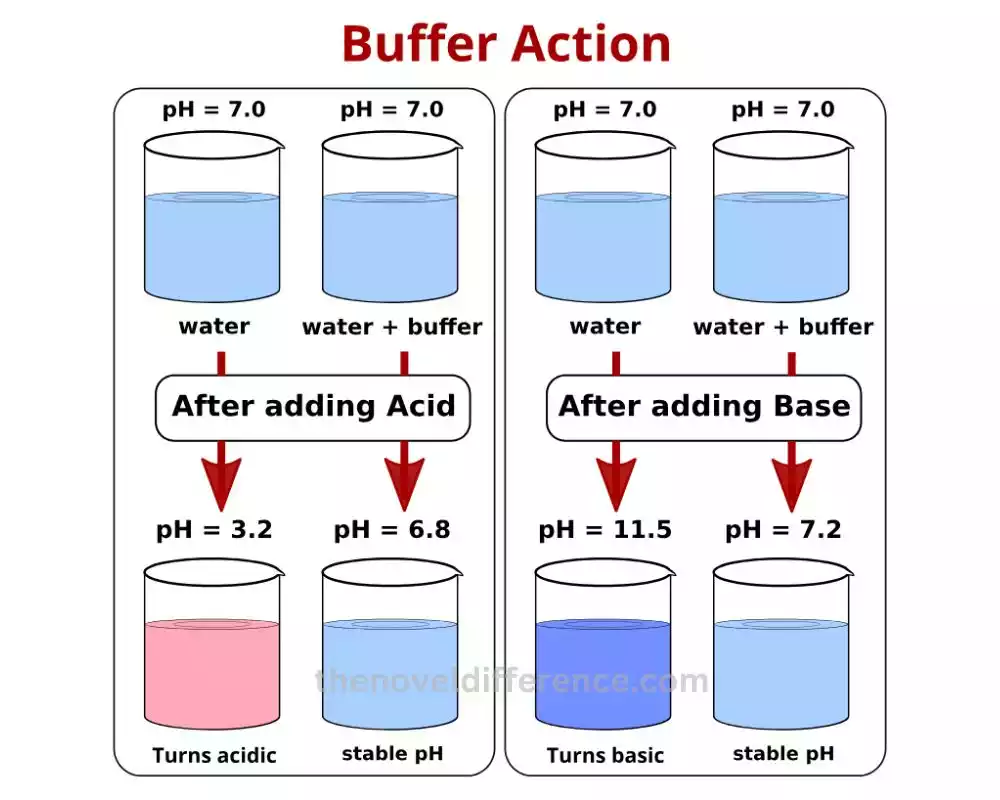
What is Buffer Capacity?
While buffer action relates to the ability of a buffer solution to resist changes in pH, buffer capacity delves into the quantification of this ability. Buffer capacity measures the amount of acid or base that a buffer solution can neutralize before the pH significantly changes.
A high buffer capacity indicates that a buffer solution can effectively handle large amounts of acid or base without causing significant shifts in pH. On the other hand, a low buffer capacity implies that a slight addition of acid or base can lead to substantial pH variations.
Buffer capacity depends on two main factors: the concentration of the buffering components and the ratio of the weak acid to its conjugate base (or weak base to its conjugate acid). Higher concentrations of buffering components and an equal ratio of weak acid to its conjugate base (or weak base to its conjugate acid) contribute to a higher buffer capacity.
Imagine a scenario where you have two buffer solutions, one with a high buffer capacity and another with a low buffer capacity. If you add the same amount of acid or base to both solutions, the one with high buffer capacity will experience only a minor change in pH, while the low-capacity solution will undergo a more significant pH shift.
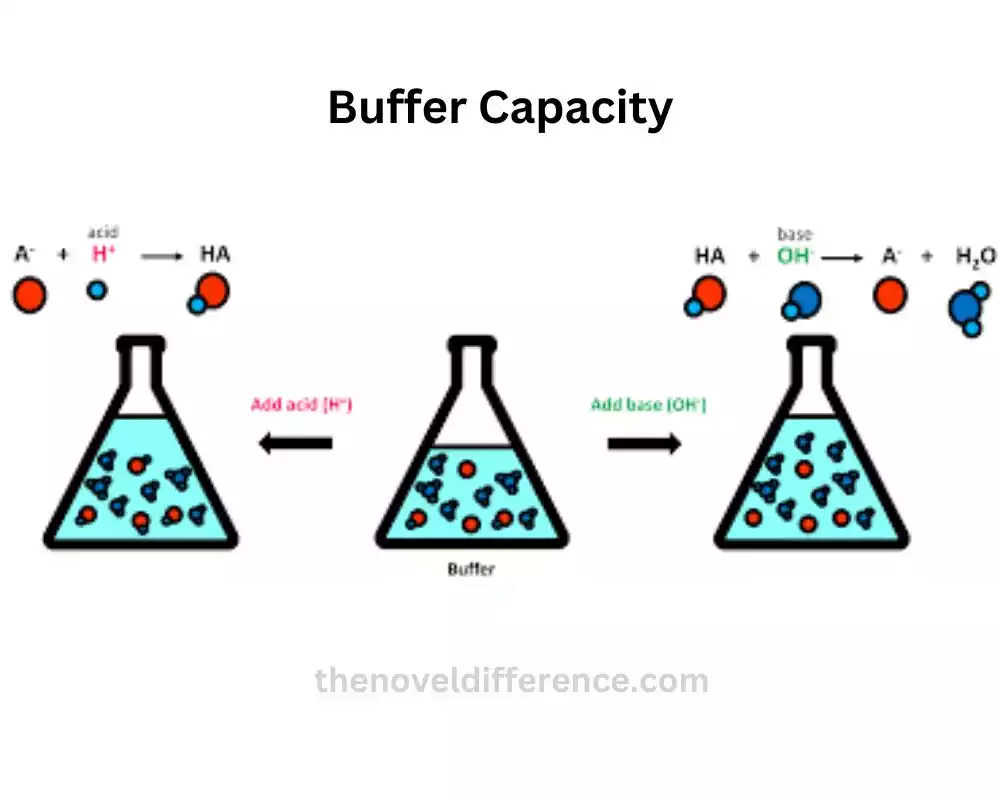
Buffer capacity plays a crucial role in maintaining stable pH conditions in various biological and chemical processes. For example, in biological systems, buffers help keep the pH in our body fluids stable, allowing enzymes and proteins to function optimally.
The Power of Buffer Action in Biological Systems
Have you ever wondered how our bodies maintain a stable pH in our blood and other bodily fluids? The answer lies in a fascinating phenomenon known as buffer action. Buffer action is the ability of a buffer solution to resist changes in pH, and it plays a vital role in ensuring our biological systems function optimally. I’ll walk you through the steps of buffer action in biological systems, so let’s dive in!
Step 1: Meet the Buffer Heroes Buffers are like the unsung heroes working behind the scenes to keep everything running smoothly. The primary buffering agents in our bodies are bicarbonate ions (HCO3-) and carbonic acid (H2CO3). They form what is known as the bicarbonate buffer system.
Step 2: The Dance of the Protons When excess acid is introduced into our blood, it brings along a bunch of hydrogen ions (H+). These sneaky little protons can wreak havoc on our pH levels if left unchecked. But fear not, for the bicarbonate buffer system steps in to save the day.
Step 3: Neutralization to the Rescue When the hydrogen ions encounter bicarbonate ions in the buffer system, they form carbonic acid. This reaction is a classic case of neutralization, where the acidic hydrogen ions combine with the basic bicarbonate ions to create a more stable compound.
Step 4: Keeping the Balance when excess base is present in our blood, it releases hydroxide ions (OH-), which can raise the pH. But the bicarbonate buffer system is always ready to restore balance. Carbonic acid dissociates, releasing bicarbonate ions and neutralizing the excess base.
Step 5: The Continuous Cycle This dance of neutralization and dissociation occurs continuously in our bodies, maintaining the pH of our blood within a narrow and optimal range. It’s like a well-choreographed performance that keeps going, ensuring the delicate balance of our biological systems.
Difference between Buffer Action and Buffer Capacity
Buffer Action:
1. Definition: Buffer action refers to the ability of a buffer solution to resist changes in pH when small amounts of acid or base are added to it.
2. Mechanism: Buffer action is based on the equilibrium between the weak acid and its conjugate base (or weak base and its conjugate acid) in the buffer solution. The buffer components react with added acid or base to neutralize them and prevent significant changes in pH.
3. Purpose: Buffer action helps maintain the pH stability of a solution within a specific range, providing a relatively constant environment for chemical reactions or biological processes.
4. Measurement: Buffer action can be quantitatively described using the Henderson-Hasselbalch equation, which relates pH, pKa, and the concentrations of the acid and its conjugate base.
Buffer Capacity:
1. Definition: Buffer capacity refers to the ability of a buffer solution to resist changes in pH when larger amounts of acid or base are added to it.
2. Mechanism: Buffer capacity depends on the concentration of the buffer components and the nature of the weak acid and its conjugate base (or weak base and its conjugate acid). Higher concentrations of buffer components and stronger acids/bases result in higher buffer capacity.
3. Purpose: Buffer capacity measures the effectiveness of a buffer in maintaining its pH stability when exposed to significant amounts of acid or base. It determines the maximum amount of acid or base that can be added before a significant change in pH occurs.
4. Measurement: Buffer capacity is calculated using the concept of moles. It is expressed as the change in the amount of added strong acid or base per unit change in pH.
Buffer action refers to the immediate response of a buffer solution to resist small pH changes, while buffer capacity measures the buffer’s overall ability to withstand larger amounts of acid or base without significant pH alterations. They are both important aspects of buffer systems and contribute to maintaining pH stability in different scenarios.
Factors Influencing Buffer Action
Have you ever wondered what makes a buffer solution effective in resisting changes in pH? It turns out that several factors come into play, influencing the buffer action and its ability to maintain stability. I’ll guide you through the key factors that influence buffer action, demystifying this essential concept. Let’s dive in!
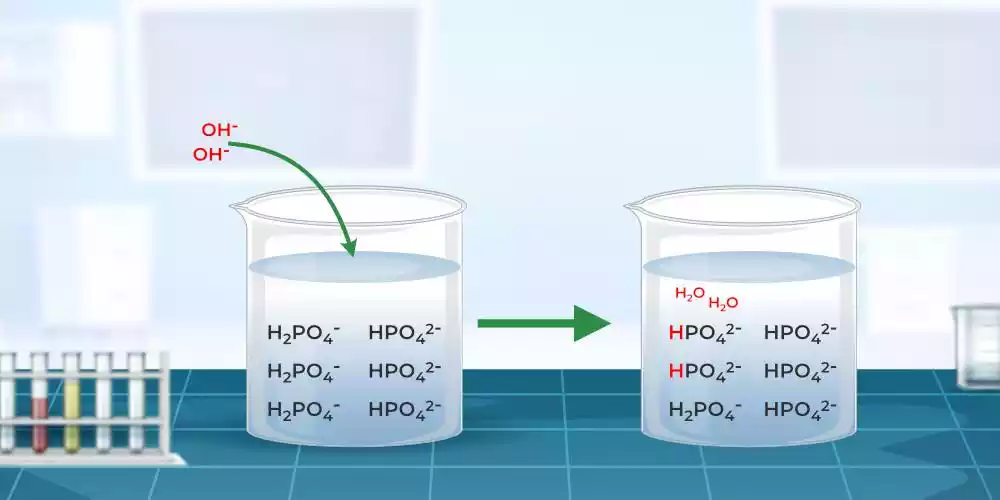
Step 1: Concentration is Key The concentration of the buffering components plays a crucial role in determining the buffer action. Simply put, the higher the concentration of the weak acid and its conjugate base (or the weak base and its conjugate acid), the more effective the buffer solution will be in resisting pH changes. So, remember, concentration is key when it comes to buffer action.
Step 2: The Ratio Matters Apart from concentration, the ratio of the weak acid to its conjugate base (or the weak base to its conjugate acid) is also significant. A buffer solution with an equal ratio of the weak acid to its conjugate base (or weak base to its conjugate acid) exhibits the maximum buffer action. This balanced ratio ensures that both the acidic and basic components work together harmoniously, resisting pH shifts effectively.
Step 3: pH Sweet Spot The pH range in which a buffer solution is most effective is known as the buffer range or the pH sweet spot. The buffer range depends on the pKa value of the weak acid (or weak base) present in the buffer system. The pKa represents the acid dissociation constant and provides insights into the strength of the acid (or base). For optimal buffer action, it is ideal to choose a weak acid (or base) with a pKa value close to the desired pH range.
Step 4: Temperature Matters To Temperature can influence the buffer action as well. Temperature changes can alter the equilibrium between the weak acid and its conjugate base (or weak base and its conjugate acid), affecting the buffer’s performance. Generally, buffer solutions are more effective at specific temperatures, so maintaining a consistent temperature can help maximize buffer action.
To summarize
Buffer action refers to the ability of a buffer solution to resist pH changes. Buffer capacity quantifies the buffer solution’s ability to neutralize acid or base without significant pH variations.
Buffer capacity depends on the concentration of buffering components and the ratio of weak acid to its conjugate base (or weak base to its conjugate acid).
Understanding the difference between buffer action and buffer capacity is vital for comprehending the behavior of buffer solutions. Buffer action refers to the ability of a buffer to resist pH changes, while buffer capacity quantifies this ability. Buffer solutions play a crucial role in maintaining stable pH conditions, ensuring the proper functioning of biological and chemical systems.
